Catheter ablation of symptomatic paroxysmal atrial fibrillation is an established therapeutic procedure with good prospects of success and low risk. Pulmonary vein isolation remains the basis of successful catheter ablation. However, a proportion of AF patients are found to have independent fibrotic atrial disease, which provides the substrate for the maintenance of AF. This particularly (but not exclusively) affects patients with persistent atrial fibrillation. This realization has paved the way for individualized ablation concepts to achieve long-term stabilization of sinus rhythm even in this challenging patient population. The targeted treatment of atrial fibrillation “modulators” (i.e., promoting factors such as arterial hypertension, obesity, sleep apnea, diabetes, etc.) should in the future be interdisciplinary.
Catheter ablation of atrial fibrillation (VHF) has become a routine procedure with good clinical results. Long-term success rates are currently 80-90% for paroxysmal atrial fibrillation (PAF) and 50-60% for persistent atrial fibrillation (PersAF) [1,2].
Catheter ablation can significantly improve patients’ quality of life. Moreover, studies not only demonstrate the superiority of catheter ablation over antiarrhythmic drugs with respect to the recurrence of VCF, but now also provide evidence that ablation may influence the prognosis for ischemic stroke and mortality [3].
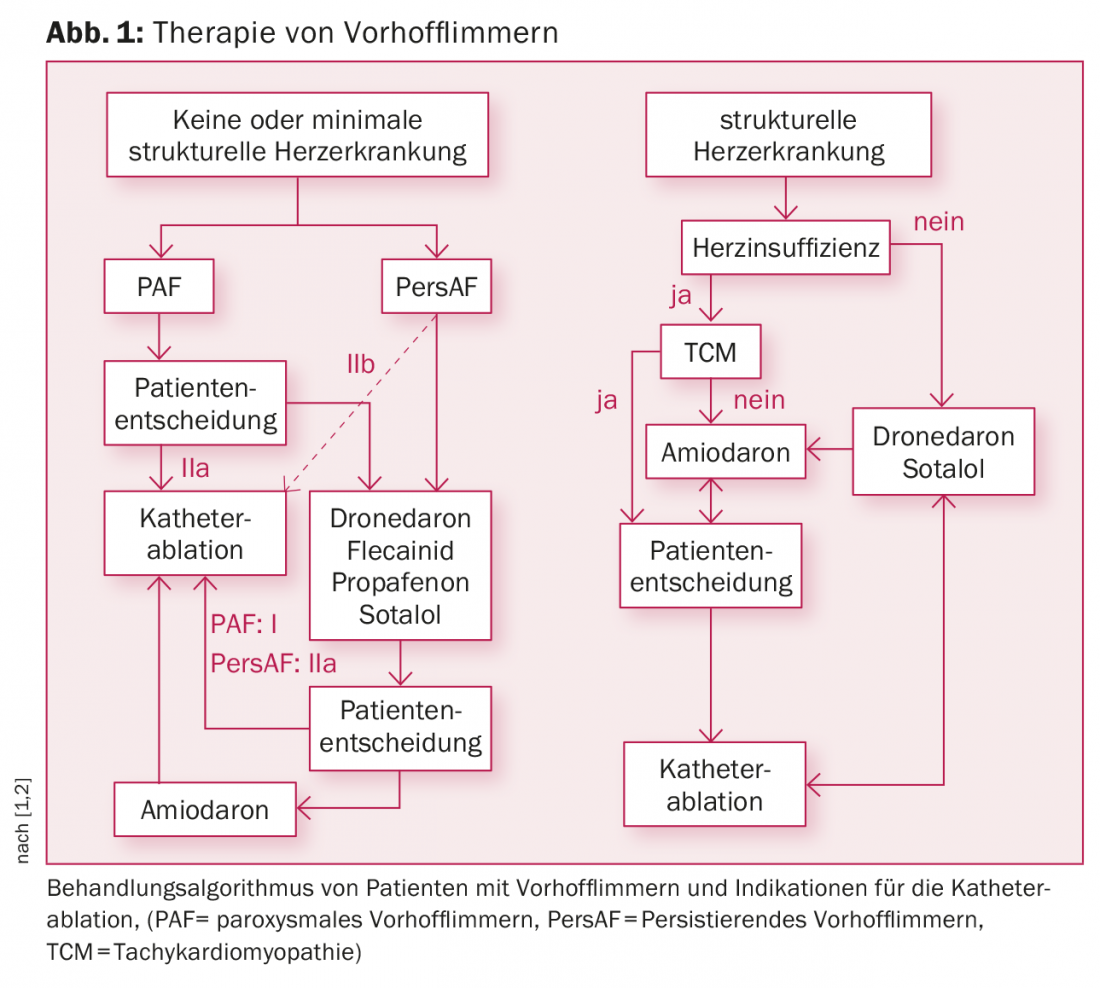
Depending on whether or not prior antiarrhythmic drugs are used, current guidelines list a class I or IIa indication for PAF and a IIa or IIb indication for persAF (Fig. 1) [1,2]. Especially for PAF, catheter ablation is already used as first-line therapy.
The pathophysiology of nonvalvular VCF has been the subject of intense investigation in recent years, with a significant increase in our knowledge of the pathophysiological background.
The role of triggers
Haissaguerre and coworkers first described the role of pulmonary vein (PV) triggers in the initiation of VCF nearly two decades ago [4]. This observation formed the cornerstone for the development of pulmonary vein isolation (PVI) as a potentially curative treatment strategy. Since then, this has been the main cornerstone of catheter ablation of VCF. Rarely, non-PV triggers play a role in initiation. Some of the latter may originate in the left atrial tube, coronary venous sinus, or interatrial septum.
The pulmonary vein isolation
PVI is virtually a component of any catheter ablation of VCF and can be achieved by using a variety of tools or techniques (Fig. 2 A-C).
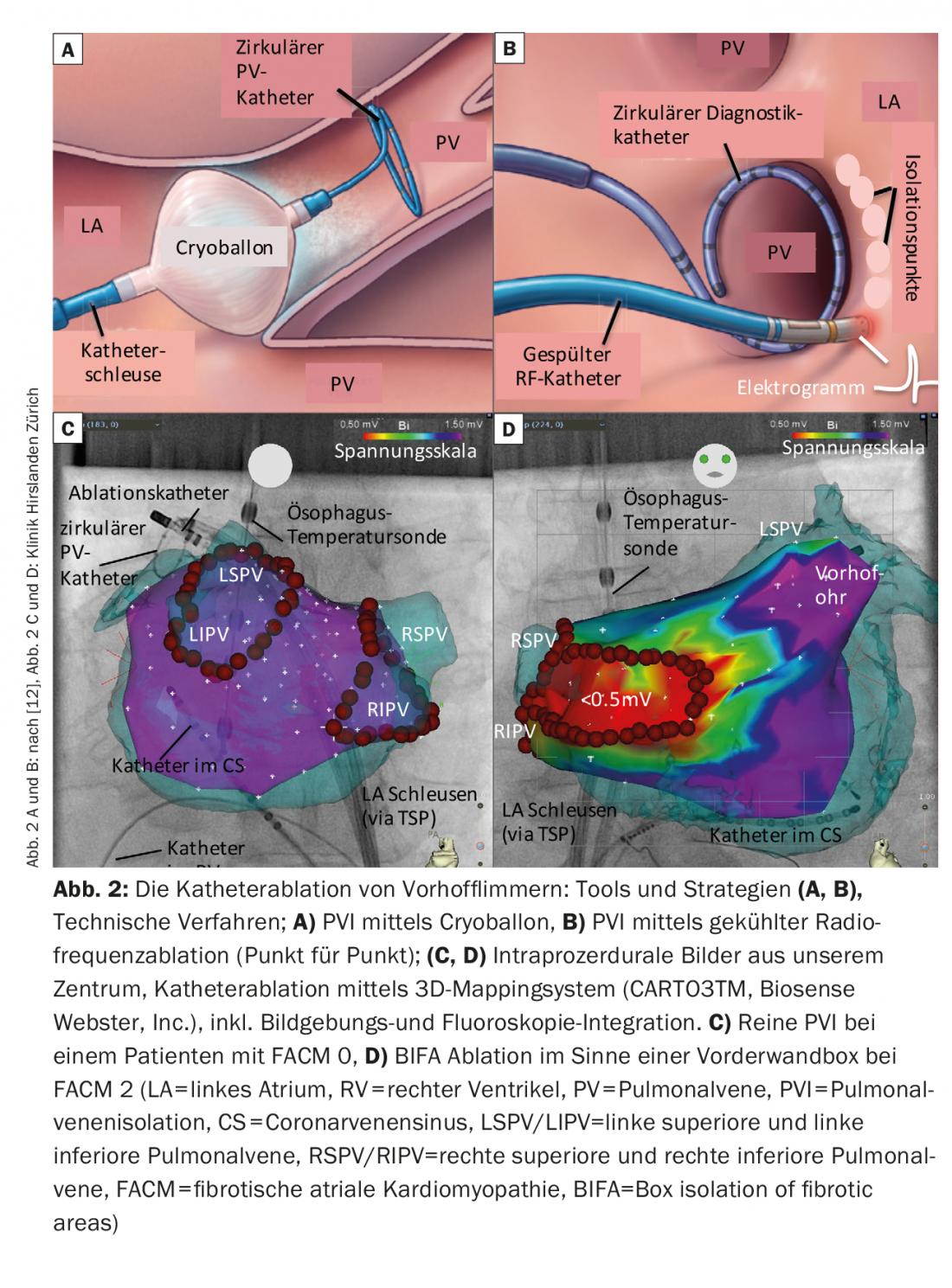
The form of energy with which the most experience is available worldwide is high-frequency current (= radio frequency, RF). A circumferential line is usually created around the ipsilateral PV by point-by-point ablation over the catheter tip. Electrical isolation is usually verified by periprocedural use of circular mapping catheters. Electroanatomic mapping systems or imaging techniques for image integration are often used (CT, MRI, intracardiac ultrasound). These facilitate navigation within the atrium, contribute to the safety of the intervention and reduce fluoroscopy times. The sustainability of ablation lesions has also improved with physical technologies such as navigable airlocks, various catheter flushing techniques, and especially measurement of catheter contact pressure. The rate of PV line recoveries could be reduced as a result.
Alternatively, in addition to RF ablation, cryoballoon ablation in particular is being used more and more frequently today. In this procedure, the 4 PVs are isolated individually, and fluoroscopy with the aid of contrast injections is used to evaluate an optimal balloon position. Balloons are often easier to handle, but have the limitation that detailed diagnosis/therapy of consecutive arrhythmias or analysis of left atrial electrograms outside the PV during the procedure is not possible.
Very rarely, laser energy is used for PVI. Experience is still limited in this regard.
In PAF, very good long-term results can usually be achieved with just one procedure using pure PVI. In contrast, despite technical and strategic advances, persAF success rates are limited, even after multiple procedures in some cases. Recurrences after ablation cannot always be explained by PV conduction recovery.
The left atrial substrate
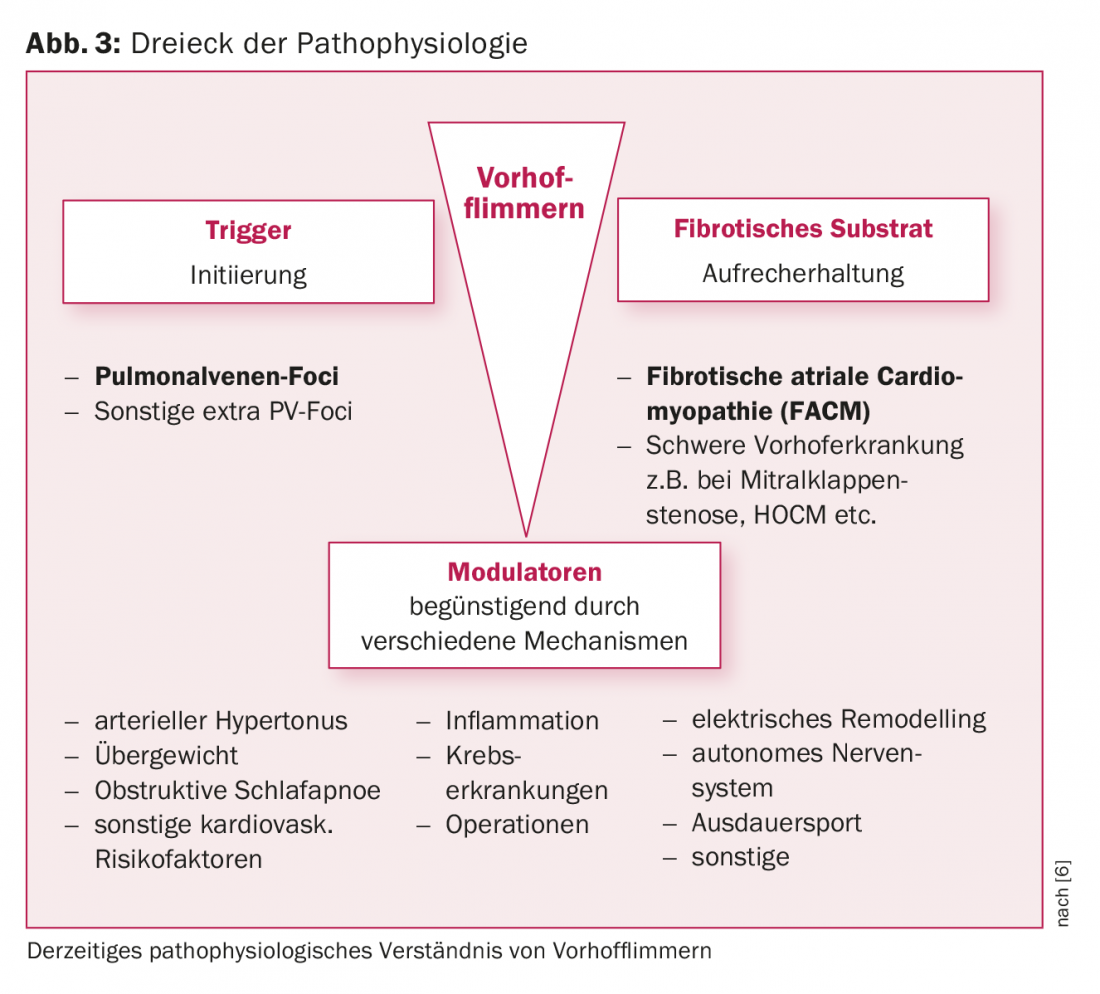
The presence of left atrial fibrosis is a typical pathological finding in patients with VHF. This left atrial substrate plays a critical role in the maintenance of VCF. The latter may develop on the floor of significant structural heart disease (e.g., mitral valve stenosis, HOCM) or may exist in the form of independent atrial disease. In this context, the concept of preexisting fibrotic atrial cardiomyopathy (FACM) has become established in recent years [5 , 6] (Figs. 3, 4). Experimental studies have shown that fibrotic structural remodeling processes lead to an electrically anisotropic situation (alteration of conduction velocities, refractory periods, cell-cell contacts), which favors the maintenance of VCF. We now know that ablation success rates depend significantly on the extent of underlying atrial fibrosis.
The creation of a bipolar voltage map during the procedure can map this left atrial substrate well (the healthy areas are color coded purple, the highly fibrotic areas <0.5 mV red).
Today, our center divides FACM into 5 stages depending on the degree of severity (Fig. 4) (FACM 0: no significant low-tension areas in the voltage map, FACM 1: local fibrosis of minor extent, FACM 2: larger, confluent fibrosis areas, FACM3: pronounced atrial fibrosis, but still regionally limited, FACM 4: almost ubiquitous fibrosis).
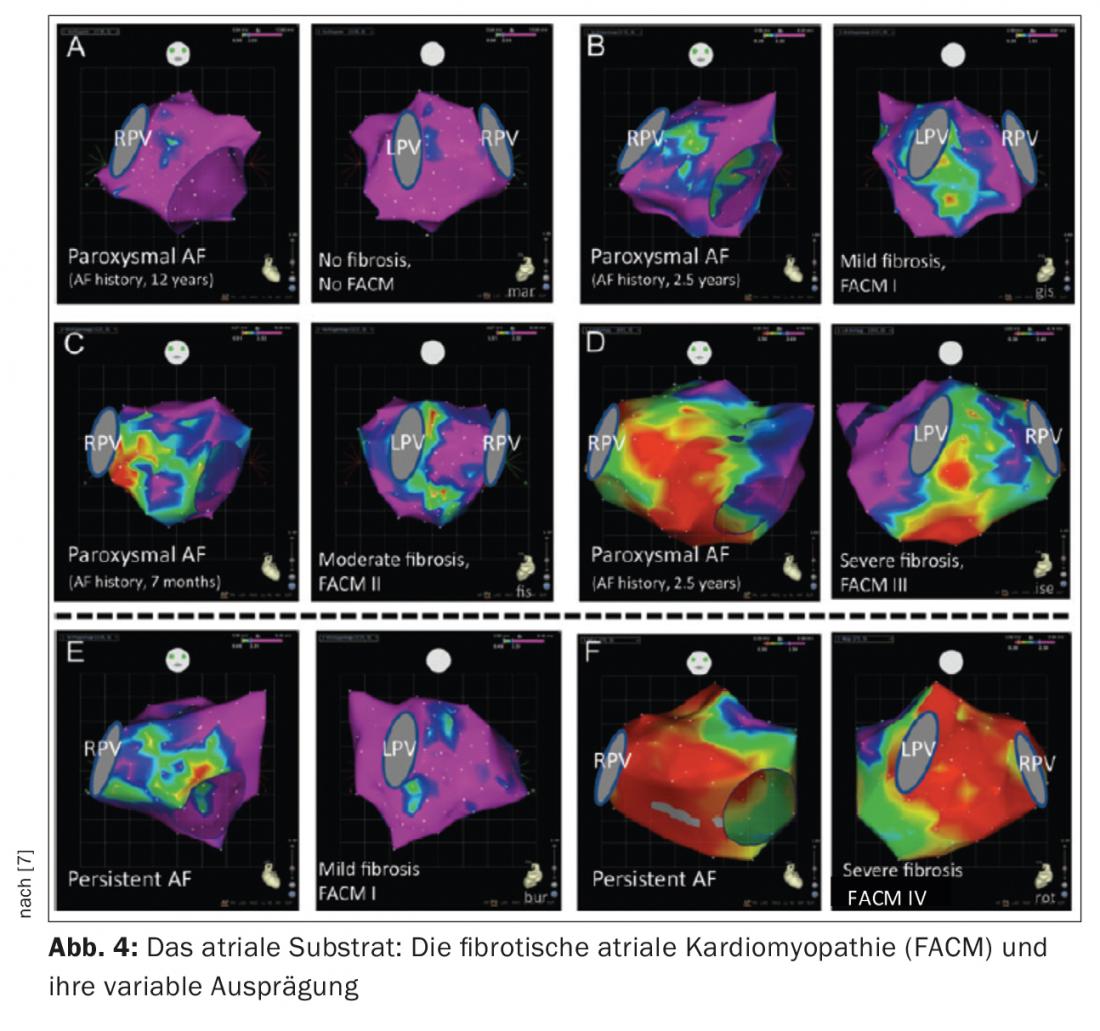
It has long been assumed that the duration and magnitude of atrial fibrillation episodes provide the critical stimulus for fibrotic remodeling of the atria (“AF begets AF”). Meanwhile, there are doubts about this theory. This is because it has been shown that patients with both PAF and persAF may have significant atrial disease. In our patients, fibrosis areas can be detected in almost 20% of PAF patients and in 70% of persAF patients.
Previous and new individualized methods for substrate modification.
PVI alone is successful in just under 50% of patients with persAF. However, previous ablation strategies that extend beyond PVI (e.g., ablation of complex fractionated electrograms, so-called CFAEs, or creation of lines, or a combination of both) have not contributed to a significant improvement in ablation outcomes. The latter has been demonstrated in particular by the prospective multicenter STAR AF II study [1,8].
Therefore, individualized ablation concepts have been developed that take into account (in addition to PVI) the underlying pathomechanisms of VCF.
First, from a functional point of view, atrial “rotors” have come into focus in the last 5 years. New electrophysiological mapping techniques make it possible to identify these “motors” of the VHF using a basket-shaped multielectrode catheter, or more rarely noninvasively by “body surface mapping,” and make them accessible to targeted ablation. However, the clinical value, particularly in patients with persAF, needs further investigation. The ablation results are controversial [9].
Among other things, there are now ablation concepts that focus on the structural changes, i.e., left atrial fibrosis. Box isolation of fibrotic areas (BIFA), which goes beyond circumferential PVI, is one such strategy developed at our center (Fig. 2B). Based on a voltage map established by the atrium in sinus rhythm, fibrotic areas, ie, “low-voltage areas” (<0.5 mV) are electrically isolated. The goal is to eliminate the diseased atrial areas and thus the potentially arrhythmogenic substrate.
Despite a difficult patient population, the BIFA concept achieves good ablation results with initial 1-year success rates around 85% with only 1.2 procedures per patient [10].
Due to the highly variable expression of FACM, an optimal ablation concept can be chosen individually during the procedure. Imaging techniques as a preliminary screening method are currently still being tested. In patients without detectable FACM (FACM 0), PVI alone appears to be sufficient, even in persAF. Here, prognostically, there is a good chance of long-term preservation of sinus rhythm (80-90%) with only 1.2 procedures/patient [10]. Catheter ablation is significantly more challenging in patients with existing atrial disease. The extent to which patients with the highest grade of atrial fibrosis (FACM 4) may benefit from catheter ablation in the long term is currently not conclusively established.
Modulators of atrial fibrillation
A number of clinical risk factors (age, hypertension, obesity, sleep apnea, diabetes, alcohol, etc.) promote the occurrence of VCF. Certain concomitant circumstances such as inflammation/infection or even a perioperative setting also favor arrhythmia recurrence (Fig. 3).
In recent years, it has been shown that targeted treatment with so-called “atrial fibrillation modulators” has a positive effect. For example, the Australian research group led by Sanders demonstrated that targeted treatment of clinical risk factors leads to a reduction in VHF-associated symptoms, induces cardiac remodeling, and can even significantly improve the outcomes of catheter ablation [11].
Concomitant risk factor management should therefore be an essential component of rhythm control in practice not only before but also after catheter ablation. Interdisciplinary treatment of patients with VHF is therefore desirable.
Complications
The potential complications of catheter ablation of VCF depend on the ablation technologies and strategies used, as well as the experience of the investigator.
The overall complication rate is approximately 4-5%, with about half attributable to peripheral vascular complications related to inguinal access [1,12,13]. Severe complications are rare.
Pericardial effusions/tamponades are reported in 1-2.5% of cases, with slightly lower rates of 0.5% for cryoballoons. The risk of cerebrovascular complications (TIA/strokes) has been reduced by measures such as preinterventional thrombus exclusion by transesophageal echocardiography, periprocedural heparinization (ACT-guided), and continuous catheter/vascular lavage in recent years and is now only about 0.5%.
PV stenoses as a thermal effect of ablation practically do not occur today due to the newer ablation strategies outside the PV orifice areas and are reported in the literature to be 0-0.3%.
Diaphragmatic paresis is a rare but typical complication of cryoballoon ablation (2-5%). However, it is often detectable only temporarily, so that after 3 months it is described in only 0.2% of cases. An extremely rare but potentially life-threatening complication is the occurrence of an atrioesophageal fistula (single case reports or approximately 0.04%). Prophylactic measures in this regard are clearly recommended: Atrial posterior wall energy reductions and the use of esophageal temperature probes that detect excessively high or low temperatures during ablation.
A look into the future
VCF is a complex and heterogeneous disease. Catheter ablation can effectively treat a large proportion of patients and establish stable sinus rhythm in the long term. However, the severity of the underlying atrial disease, which does not correlate with the clinical presentation (PAF vs. persAF) in every case, is of crucial importance for the long-term prognosis.
Individualized “tailor-made” ablation concepts are therefore crucial to achieve optimal results. New diagnostic, ideally high-resolution, automated mapping techniques during catheter ablation will give us further insight, particularly into the mechanisms of maintenance of VHF. In addition, the importance of imaging techniques will increase (e.g., specific echo analyses such as strain, cardiac MRIs, etc.) with the potential to decide on adequate therapies or specific ablation concepts in advance of the procedure. Furthermore, in the future, biomarkers (eg, ex-tracellular matrix markers) or known genetic predispositions may provide midterm clues to the extent and progression of atrial substrate.
Literature:
- Calkins H, et al: HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012; Apr;14(4): 528-606.
- Kirchhof P, et al: 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS: The Task Force for the management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESCEndorsed by the European Stroke Organisation (ESO). Europace. 2016 Aug 27 [Epub].
- Friberg L, et al: Catheter ablation for atrial fibrillation is associated with lower incidence of stroke and death: data from Swedish health registries. Eur Heart J. 2016; Mar 16. pii: ehw087. [Epub ahead of print]
- Haïssaguerre M, et al. : Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998; Sep 3;339(10): 659-66.
- Kottkamp H, et al: Catheter ablation of atrial fibrillation: how to modify the substrate? J Am Coll Cardiol. 2015; Jan 20;65(2): 196-206.
- Kottkamp H, et al: The Substrate in “Early Persistent” Atrial Fibrillation Arrhythmia Induced, Risk Factor Induced, or From a Specific Fibrotic Atrial Cardiomyopathy? JACC CEP 2016; 2: 140-142.
- Kottkamp H: Human atrial fibrillation substrate: toward a specific fibrotic atrial cardiomyopathy. Eur Heart J. 2013; Sep;34(35):2731-8.
- Verma A, Jiang CY, et. al: STAR AF II Investigators. Approaches to catheter ablation for persistent atrial fibrillation. N Engl J Med. 2015; May 7;372(19): 1812-22.
- Guillem MS, Climent AM, et al: Presence and stability of rotors in atrial fibrillation: evidence and therapeutic implications. Cardiovasc Res. 2016, 109(4): 480-92. [Epub].
- Kottkamp H, et. al: Box Isolation of Fibrotic Areas (BIFA): A Patient-Tailored Substrate Modification Approach for Ablation of Atrial Fibrillation. J Cardiovasc Electrophysiol. 2016; Jan27(1):22-30.
- Pathak RK, et al: Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study. J Am Coll Cardiol 2014;64: 2222-2231.
- Kuck KH, et al: Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016; 374: 2235-45.
- Shah RU, et al: Procedural complications, and repeat procedures After Catheter Ablation for Atrial Fibrillation. J Am Coll Cardiol 2012; 59: 143-9.
CARDIOVASC 2016; 15(6): 3-8