Chronic kidney disease (CKD) is classified according to the type of underlying disease, filtration rate and extent of proteinuria. Patients with CKD are cardiovascular risk patients. Cardiovascular morbidity increases with the increase in proteinuria and with the decrease in filtration rate. The therapeutic basis for slowing the progression of renal failure remains antiproteinuric therapy by inhibiting the RAS. Concerning arterial and renovascular hypertension: it tends to be advisable not to use too intensive blood pressure control. Renovascular hypertension is the most common cause of secondary hypertension in patients older than 50 years with resistant hypertension and is associated with higher cardiovascular mortality and more rapid renal function loss. Renal denervation should not be used as a standard method of treating patients with “refractory” hypertension.
The first part of this article focuses on chronic renal failure. How does the diagnosis progress and what is there to say about the risk of progression?
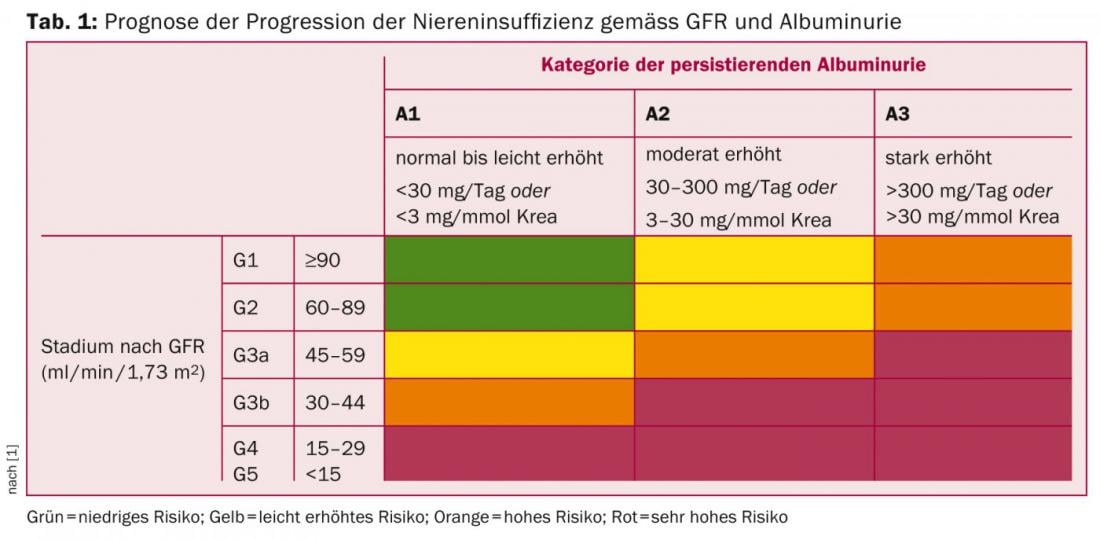
Chronic kidney disease (CKD) is defined by a filtration rate (eGFR) ≤60 ml/min/1.73 m2 or by signs of renal damage (in urine, serum, or by imaging) over three months, according to the 2012 KDIGO guidelines [1]. Classification of CKD should be sought according to type of underlying disease, filtration rate, and extent of proteinuria [1], because these parameters are relevant not only for progression (Table 1) but also for estimating cardiovascular morbidity and mortality.
The estimation of eGFR should take place in everyday life by the 2009-CKD-EPI formula [1]. Cystatin C together with serum creatinine for eGFR determination (2012-CKD-EPI creatinine-cystatin C formula) should be used in special situations [2]. This is especially true when serum creatinine does not correlate with eGFR (e.g., muscle loss, liver cirrhosis, pregnancy, trimethoprine use) and when there is no other evidence of renal disease other than decreased GFR estimated by serum creatinine [1]. Both formulas are available on the Internet at www.mdrd.com.
The presence of microalbuminuria is an unrelated cardiovascular risk factor [3], although it is not always associated with progression of renal disease, as microalbuminuria can also be regressive during its course [4]. The decrease in filtration rate in elderly patients down to CKD 3a (eGFR 45 ml/min/1.73 m2) can be considered a normal aging process in the presence of stable eGFR and absence of proteinuria [1].
Cardiovascular complications (prevention)
Patients with CKD are cardiovascular risk patients. Cardiovascular morbidity increases with the increase in proteinuria and with the decrease in filtration rate. The effect appears to be additive [5]. Risk factors can be divided into traditional (age, LDL cholesterol, hypertension, sex, etc.) and nontraditional (albuminuria, anemia, volume load, mineral-bone balance disorder). The new KDIGO guidelines recommend moderate lowering of LDL cholesterol with a fixed dose of a statin after a single measurement of cholesterol in patients over 50 years of age not requiring dialysis, without further monitoring of the lipid profile according to the “fire and forget” strategy [6]. In patients younger than 50 years, drug therapy is not recommended until coronary artery disease, diabetes mellitus, stroke, or a calculated 10-year risk of coronary events >10%. In this case, the target values of the underlying disease apply [6].
Progression slowing of renal failure.
Antiproteinuric therapy by inhibition of the renin-angiotensin-aldosterone system (RAS) remains the cornerstone of slowing the progression of renal failure. Since more than one class of compounds is available to inhibit the RAS, the extent to which dual RAS blockade has benefits in terms of cardiovascular mortality and progression to renal failure is being investigated.
The ONTARGET trial is the largest study to date that has evaluated the combination of an ACE inhibitor with a sartan against ACE inhibitor or sartan treatment alone in high-risk patients. Combination therapy showed no benefit in cardiovascular mortality. Regarding renal endpoints, combination therapy even showed a worse outcome [7]. A major drawback of ONTARGET was that few patients with proteinuria were included. Here, the ALTITUDE study [8] (combination of the renin inhibitor aliskiren with ACE-I or ARB) and most recently the NEPHRON-D study [9] (combination of losartan with lisinopril vs. losartan alone) in patients with type 2 diabetes and proteinuria showed that serious side effects (hyperkalemia, acute renal failure) occurred more frequently with combination therapy, so that the studies were terminated prematurely. Double RAS blockade is no longer recommended in patients with diabetes and proteinuria. It remains unclear whether patients with proteinuric nephropathy due to glomerulonephritis benefit from dual RAS blockade.
Calcium-phosphate balance and disturbance of the mineral-bone balance
The pathophysiology of mineral and bone balance disorders is classically perceived as developing secondary hyperparathyroidism due to progressive calcitriol deficiency and phosphate retention. New findings on the FGF-23/Klotho system have changed this perception.
Even in early stages, there is an increase in FGF-23, which simultaneously inhibits calcitriol and parathyroid hormone synthesis [10]. It is possible that in the early stages of renal failure, FGF-23 regulates a positive phosphate balance that is invisible in serum. At the same time, FGF-23 serves to keep the calcium balance stable. With the further loss of renal output, the effect of the FGF-23 co-receptor Klotho decreases. Parathyroid hormone and FGF-23 then rise in parallel. However, the exact pathophysiology remains unclear.
According to the KDIGO guidelines, normal values for serum phosphate and parathyroid hormone should be aimed for in patients not requiring dialysis [11]. The use of phosphate binders in patients with advanced renal failure who are not on dialysis has been questioned recently [12,13]. Based on the above data, clinical practice should focus on reducing phosphate intake as early as possible – the importance of nutritional counseling with the goal of phosphate reduction is increasing [1]. However, one must avoid achieving phosphate reduction by protein restriction and malnutrition [1]. In case of vitamin D deficiency (25[OH]vitamin D <50 nmol/l), which is often present in CKD patients [14], substitution is recommended.
Arterial and renovascular hypertension
The second part of this article is about arterial and renovascular hypertension: what are the current blood pressure targets? And what news is there in this area?
Guidelines for the treatment of arterial hypertension have changed in recent years. In 2012, the KDIGO guidelines were published, specifically addressing blood pressure control in non-dialysis patients with chronic renal failure. Target blood pressures have been raised for patients without albuminuria (<30 mg/day) with the target blood pressure <140/90 mmHg. However, for patients with albuminuria, aggressive targets are maintained without hard evidence [15].
In 2013, the ESH/ESC guidelines were updated (Tables 2 and 3), in which systolic blood pressure reduction to <140 mmHg is advised in all patients at high cardiovascular risk, including those with manifest proteinuria (with and without diabetes) [16].

Diastolic blood pressure on therapy should be less than 90 mmHg. Only in patients with diabetes is a further reduction to 85 mmHg recommended [16]. The current tendency is to advise against too intensive blood pressure lowering. This is particularly because of studies in type 2 diabetics at high cardiovascular risk, who had higher cardiovascular mortality with intensive blood pressure control [17,18].

Renovascular hypertension
Renovascular hypertension is the most common cause of secondary hypertension in patients older than 50 years with resistant hypertension (up to 45%). The prevalence of renovascular hypertension is 1-5% of patients with hypertension [19] and it is associated with higher cardiovascular mortality and faster renal function loss. Large randomized trials (STAR and ASTRAL) failed to show therapeutic benefit of renal artery stenting [20,21]. These results have now been confirmed by the CORAL study, the largest randomized trial in the field to date [22]. Stent implantation also showed no benefit in subgroup analysis in patients with higher-grade stenosis (>80%), impaired renal function (eGFR <45 ml/min/1.73 m2), diabetes mellitus, bilateral stenosis, or basal elevated blood pressure (>160 mmHg). CORAL was the first trial in which many patients treated in the conservative arm received a combination of aspirin, ACE inhibitors or ARBs, and statins. A criticism of the CORAL trial is that the hemodynamic relevance of the stenosis was determined only by angiography and was not confirmed by intra-arterial pressure measurement. Furthermore, no duplex sonographic resistance index measurement had been performed, which might indicate the relevance of stenosis as the cause of hypertension [23]. A decision for stent implantation in cases of proven hemodynamically relevant renal artery stenosis should be made only after comprehensive duplex sonographic as well as clinical evaluation by angiologists and nephrologists.
Resistant hypertension
Calhoun et al. addressed the problem of refractory hypertension in a large collective (REGARDS study). After modification of therapy, exclusion of secondary causes, and control of patient adherence, only 0.5% had refractory hypertension [24]. Renal denervation has led to great debate in recent years, following the positive results of the Simplicity-1- [25] and Simplicity-2 studies [26]. The study by Fadl et al. (a small study that required assured medication adherence) questioned the efficacy of renal denervation [27]. Therefore, the results of the Simplicity-3 trial (FDA pivotal trial) were eagerly awaited (denervation vs. Sham arterial puncture and insertion of a catheter without denervation). The study showed no significant advantage of the intervention over Sham treatment [28]. Thus, renal denervation may not be used at this time as a standard method of treating patients with “refractory” hypertension.
Prof. Dr. med. Michael Dickenmann
Literature:
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidn Int Suppl 2013 3(1).
- Shlipak MG, et al: N Engl J Med 2013 Sep 5; 369(10): 932-943.
- Go AS, et al: N Engl J Med 2004 Sep 23; 351(13): 1296-1305.
- Glassock RJ, Winearls C: Curr Opin Nephrol Hypertens 2010 Mar; 19(2): 123-128.
- Matsushita K, et al. (Chronic Kidney Disease Prognosis Consortium): Lancet 2010 Jun 12; 375(9731): 2073-2081.
- KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int 2014 Feb 19. doi: 10.1038/ki.2014.31.
- Mann JF, et al: Lancet 2008 Aug 16; 372(9638): 547-553.
- Parving HH, et al: N Engl J Med 2012; 367: 2204-2213.
- Fried LF, et al: N Engl J Med 2013; 369: 1892-1903.
- Isakova T, et al: Kidney Int 2011 Jun; 79(12): 1370-1378.
- KDIGO Clinical Practice Guideline for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2009 Aug; 113: 1-130.
- Hill KM, et al: Kidney Int 2013 May; 83(5): 959-966.
- Block GA, et al: J Am Soc Nephrol 2012 Aug; 23(8): 1407-1415.
- LaClair RE, et al: Am J Kidney Dis 2005; 45: 1026-1033.
- KDIGO Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney International Supplements 2012; 2.
- Mancia G, et al: Journal of Hypertension 2013, 31: 1281-1357.
- Cooper-DeHoff RM, et al: JAMA 2010 Jul 7; 304(1): 61-68.
- Cushman WC, et al: N Engl J Med 2010 Apr 29; 362(17): 1575-1585.
- Dworkin LD, Cooper CJ: N Engl J Med 2009 Nov 12; 361(20): 1972-1978.
- Wheatley K, et al. (ASTRAL Investigators): N Engl J Med 2009; 361: 1953-1962.
- Bax L, et al: J Nephrol 2003; 16: 807-812.
- Cooper CJ, et al: N Engl J Med 2014; 370: 13-22.
- Radermacher J, et al: N Engl J Med 2001 344: 410-417.
- Calhoun DA, et al: Hypertension 2014 Mar; 63(3): 451-458.
- Symplicity HTN-1 Investigators: Hypertension 2011 May; 57(5): 911-917.
- Esler MD, et al: Circulation 2012 Dec 18; 126(25): 2976-2982.
- Fadl Elmula FE, et al: Hypertension 2014 May; 63(5): 991-999.
- Bhatt DL, et al. (SYMPLICITY HTN-3 Investigators): N Engl J Med 2014 Apr 10; 370(15): 1393-1401.
CARDIOVASC 2014; 13(4): 16-20