At the ASH Congress in New Orleans, one of the topics was the CLL11 Phase 3 trial. After showing in the last issue of InFo ONCOLOGY & HEMATOLOGY that the combination of chlorambucil with GA101 or rituximab resulted in a significant prolongation of progression-free survival compared with monotherapy, we now turn to a direct comparison of the two drug combinations.
(ag) In the last issue of InFo ONCOLOGY & HEMATOLOGY, the update of the stage 1 analysis of the CLL11 trial was presented. This phase III trial, which investigated first-line chemoimmunotherapy in patients with chronic lymphocytic leukemia (CLL) and comorbidities, was the topic of discussion at the ASH Congress in New Orleans. The results were presented by Valentin Goede, MD, University Hospital Cologne.
Patients with CLL who had no prior treatment and a total Cumulative Illness Rating Scale (CIRS) score of >6 and/or an estimated creatinine clearance of <70 mL/min were eligible for the study. They received either chlorambucil (Clb) alone (6 cycles of 0.5 mg/kgKG orally on days 1, 15, and every 28 days), obinutuzumab (GA101) plus Clb (GClb, 100 mg i.v. on day 1, 900 mg i.v. on day 2, 1000 mg on days 8 and 15 in the first cycle; 1000 mg on day 1 in cycles 2-6) or rituximab plus Clb (RClb, 375 mg/m2 i.v. on day 1 in the first cycle; 500 mg/m2 on day 1 in cycles 2-6).
What were the objectives of the study?
“The primary endpoint was progression-free survival. Important secondary endpoints to assess efficacy were response rates, minimal residual disease and overall survival,” Dr. Goede summarized.
GA101 superior with respect to important endpoints
Patients were followed for a median of 19 months. Table 1 summarizes the main results of the Level 2 analysis [1].
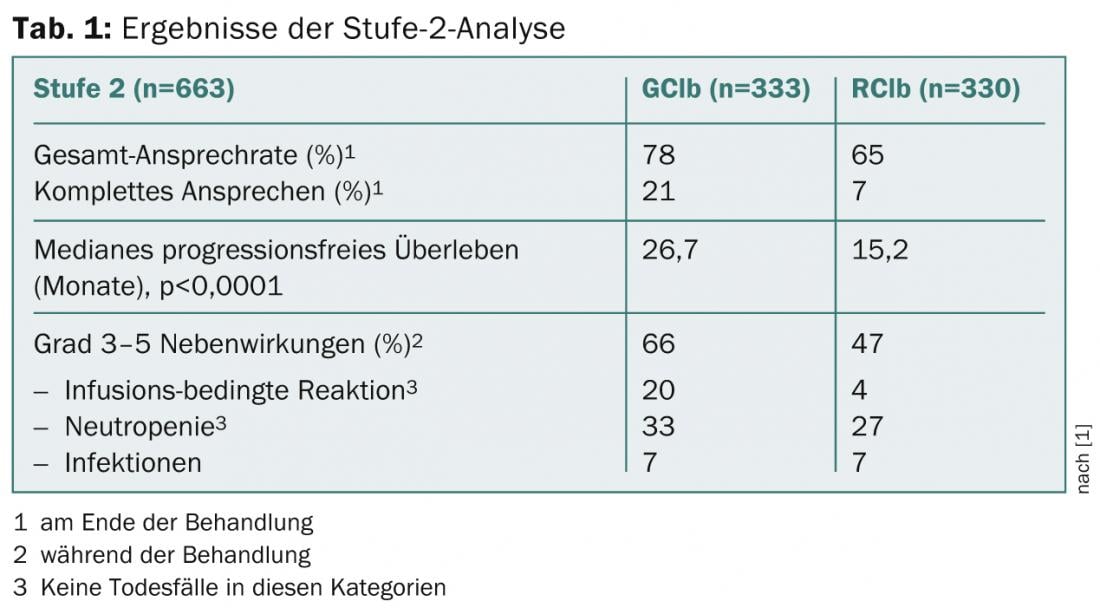
The two arms (GClb and RClb) were well balanced with respect to baseline characteristics. The median age was 73 years, the CIRS score classifying comorbidities was eight, and creatinine clearance was 63 ml/min.
All pre-planned subgroup analyses, including those on the 17p-, 11q-, 12+, 13q- cytogenetic subgroups, confirmed the significant superiority of GClb over RClb in terms of progression-free survival.
“Furthermore, it was shown that the number of patients without minimal residual disease was significantly higher in the GClb group, more than tenfold. While 29.4% reached this stage in the GA101 group, only 2.5% did so in the rituximab arm. Infusion-related reactions and neutropenias occurred more frequently under GClb, but grade 3-4 reactions occurred only with the first infusion. Infections were not more frequent. As shown in the update of the stage 1 analysis, overall survival prolonged for this compared with Clb alone. Overall, therefore, the combination with GA101 is superior to that with rituximab in this specific CLL population,” Dr. Goede concluded.
Source: 55th ASH Annual Meeting, December 7-10, 2013, New Orleans.
Literature:
- Goede V, et al: Head-To-Head Comparison Of Obinutuzumab (GA101) Plus Chlorambucil (Clb) Versus Rituximab Plus Clb In Patients With Chronic Lymphocytic Leukemia (CLL) And Co-Existing Medical Conditions (Comorbidities): Final Stage 2 Results Of The CLL11 Trial. ASH Abstract #6.
InFo Oncology & Hematology 2014; 2(2): 32-33.











