A review summarized the results from four randomized placebo- or reference-controlled double-blind trials of a Sabal-Urtica combination preparation in men over 50 years of age with BPH-associated LUTS. The phytopreparation proved to be a valid alternative to synthetic drugs. The favorable side effect profile is a plus especially for long-term therapy.
Approximately half of men over the age of 50 experience benign tissue changes in the prostate in the form of an increase in connective tissue, muscle and/or epithelial cells (benign prostatic hyperplasia, BPH). Due to organ hyperplasia, compression of the urethra may result in increased bladder outlet resistance – benign prostatic obstruction (BPO). BPH is the main cause of lower urinary tract symptoms ( LUTS), which subsumes bladder storage, voiding, and micturition symptoms [1]. In particular, α1-adrenoceptor antagonists, 5α-reductase inhibitors, and herbal therapeutics are used as first-line therapy for BPH-associated LUTS symptoms [2].
There is increasing interest in phyotherapeutic treatment options, not least because some patients are dissatisfied with the adverse effects of synthetic drugs [3]. For example, therapy with α1-adrenoceptor antagonists and 5α-reductase inhibitors may be associated with sexual side effects such as erectile dysfunction and ejaculatory dysfunction [4]. Furthermore, a relatively high risk of vascular-related adverse events is generally reported for most α-adrenoceptor antagonists [5]. Cardiovascular side effects such as hypotension, accompanied by dizziness or syncope, mainly affect older men – i.e. the main group of LUTS/BPH patients requiring drug therapy [6–8].
The drug PRO 160/120 (Prostaplant®-F) is a promising phyotherapeutic alternative for the treatment of LUTS. The combination preparation contains 160 mg extract of saw palmetto fruit (Serenoa repens or Sabal serrulata) and 120 mg dry extract of stinging nettle root (Urtica dioica) as the main pharmacological active ingredients ( box ) [9].
| Mechanisms of action of PRO 160/120 The phytopharmacological combination preparation of Sabal (saw palmetto extract) and Urtica (stinging nettle root) extracts exhibits synergistic effect. Among other things, it induces a conversion of testosterone to dihydrotestosterone and aromatase activity [18]. Relaxation of the swollen prostate tissue results in a reduction of pressure on the urethra. Due to its anti-inflammatory effect PRO 160/120 reduces irritation of the prostate [19]. Saw palmetto extract has been shown to inhibit 5α-reductase without exhibiting androgen-binding activity [20,21] and causes noncompetitive inhibition of human α1-adrenoceptors in vitro [22]. It also selectively inhibits muscarinic receptors in the lower urinary tract [23,24]. Nettle root extract inhibits Na+, K+-ATPase activity of the prostate membrane, which may limit prostate cell metabolism and growth [25]. In addition, antiproliferative, antiphlogistic, and antiedematous effects have been reported for both agents [27,28]. |
International Prostate Symptom Score (I-PSS)
In the studies included in the review, symptom improvement was assessed using the International Prostate Symptom Score (I-PSS) [10–13]. This is a commonly used reliable and valid measurement tool to evaluate BPH-associated LUTS, with seven questions about micturition each scored from 0 to 5 [2,14]. The questions usually refer to the previous month. In each of the four studies [10–13], an improvement of more than three points was achieved in the I-PSS total score, which is classified as a clinically significant change [16]. The following parameters were collected as additional endpoints: Urine time volume or urine time volume, urine flow time, mean urine flow, maximum urine flow, micturition volume, micturition duration (bladder emptying time), residual urine volume, prostate volume, quality of life. In addition, adverse events and laboratory diagnostic safety signals were recorded.
Important study results at a glance
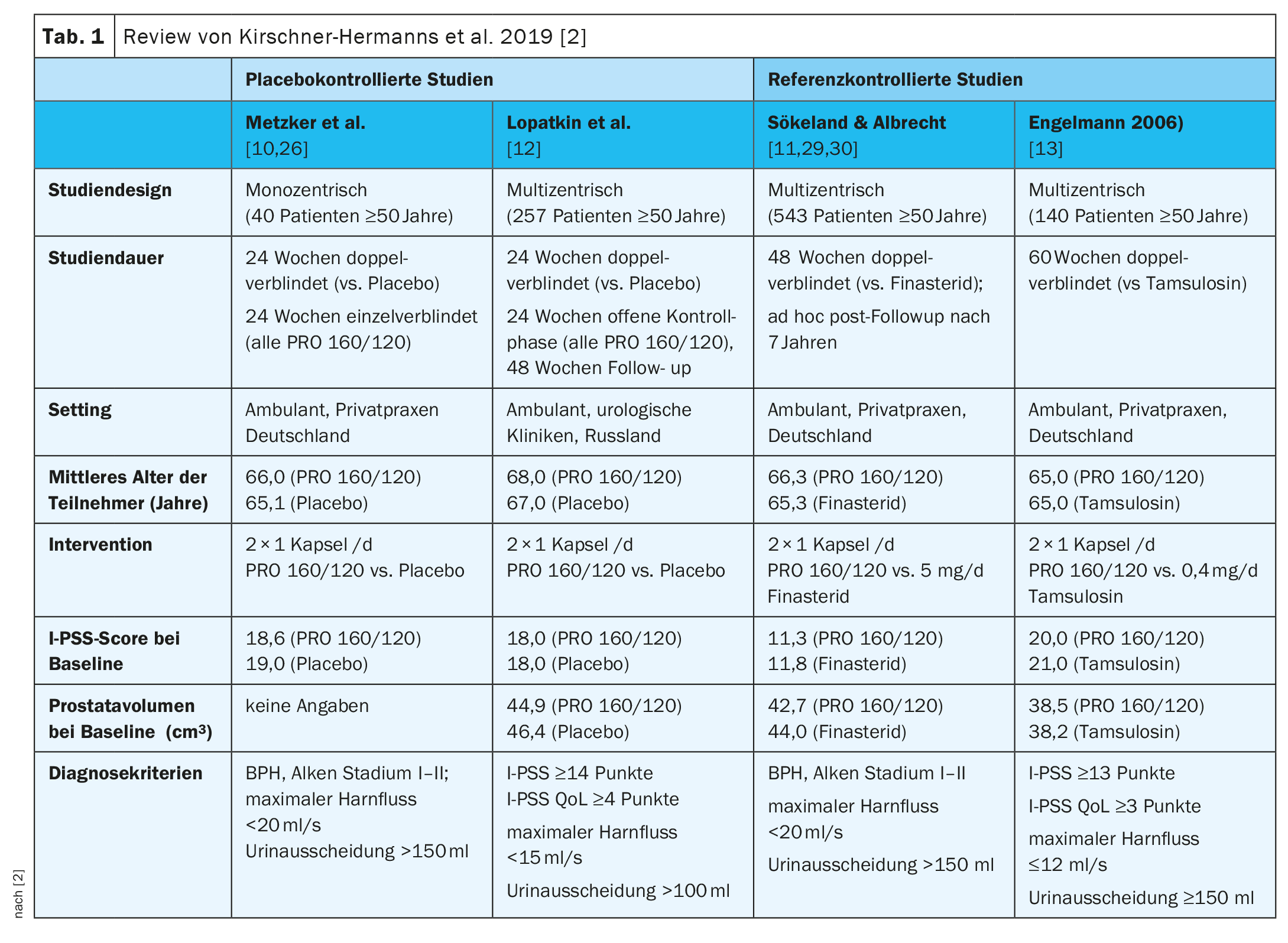
The key data of the four studies can be seen in Table 1 [2,10–13]. In Metzker et al. and Lopatkin et al. were placebo-controlled and Engelmann et al. and Sökeland & Albrecht for reference-controlled studies [2,10–13].
Metzker et al (n=40) [10]: In the double-blind treatment phase, mean I-PSS decreased from baseline values of 18.6 points in the PRO 160/120 group and 19.0 points in the placebo group to 11.1 and 17.6 points at week 24, respectively (p=0.002; two-sided U-test). In a post-hoc evaluation of these results, which focused on the I-PSS items assessing the so-called irritative symptoms, it was shown that there was a statistically significant improvement in urinary urgency and micturition frequency under PRO 160/120 during this treatment phase [26]. In the subsequent single-blinded treatment phase, I-PSS also decreased in those patients who had been switched from placebo to treatment with PRO 160/120. However, at week 48, there was still an advantage in favor of the group who were in the active treatment arm (PRO 160/120) in both phases of the study (p=0.009; two-sided U-test).
Lopatkin et al (n=257) [12]: After the double-blinded treatment phase, a statistically significant group difference was observed in the decrease of I-PSS in favor of PRO 160/120; -6 points in the treatment arm and -4 points in the placebo arm (p<0.01, one-sided U-test stratified). At the end of the control phase, the I-PSS decreased by a further 2 points in the former placebo patients (who were now also treated with PRO 160/120) and by a further point in the patients who had been treated with PRO 160/120 since the start of the study. This difference between the two treatment groups was also statistically significant (p=0.01, one-tailed stratified U test). At the end of the control phase, the reduction in mean I-PSS in both treatment groups was seven points compared with baseline, indicating that the former placebo patients benefited from PRO 160/120 treatment to the same extent as the patients who had already been treated with PRO 160/120 since the beginning of the double-blind treatment [15]. After the control phase, 213 patients (PRO 160/120: 106; placebo: 107) participated in the subsequent open-label extension of the study (weeks 49-96) [17]. In patients without missing baseline I-PSS scores (PRO 160/120: 106; placebo: 103), an additional median score reduction of 1 point was observed in each group, resulting in an overall median reduction of 9 points in both groups compared with baseline. This represents a 52.9% reduction in total I-PSS score compared with baseline.
Sökeland & Albrecht (n=543) [11]: At the end of the study, the symptomatology of patients treated with PRO 160/120 had improved to a similar extent compared with patients on finasteride therapy, with the I-PSS increasing from 11.3 ± 6.5 points (PRO 160/120) or 11.8 ± 6.6 points (finasteride) at baseline to 8.2 ± 5.8 points (PRO 160/120) and 8.0 ± 5.7 points (finasteride) at week 24 and 6.5 ± 5.8 points (PRO 160/120) and 6.2 ± 5.2 points (finasteride) at week 48 (means ± SD). The improvement in quality of life according to AUA score C was also similar compared with finasteride. The increase in maximal urinary flow under PRO 160/120 (+1.9 ml/s) and finasteride (+2.4 ml/s) proved to be therapeutically comparable within an equivalence range of ±1.5 ml/s (p=0.037; modified t-test for shifted hypotheses).
Engelmann et al. (n=140) [13]: The IPSS total score had improved by an average of 9 points at the end of treatment in both groups from a baseline score of about 20 points. A total of 32.4% of patients treated with PRO 160/120 and 27.9% of patients treated with tamsulosin were responders (i.e., IPSS total score ≤7 at the end of treatment; p=0.034, Farrington-Manning test for noninferiority; noninferiority threshold 10%). In a subgroup analysis by baseline I-PSS score, PRO 160/120 and tamsulosin were comparably effective in both patients with moderate symptoms (baseline I-PSS ≤19 points) and patients with severe symptoms (baseline I-PSS ≥20 points). Patients’ quality of life improved by a median of 2 points in the PRO 160/120 group and by 1 point in the tamsulosin group (baseline values: 3 points and 4 points, respectively; medians).
Literature:
- S2e Guideline Diagnostics and Therapy of Benign Prostate Syndrome (BPS), Registry Number: 043-034, Long Version 5.0 – February 2023.
- Kirschner-Hermanns R, Funk P, Leistner N: WS PRO 160 I 120 mg (a combination of sabal and urtica extract) in patients with LUTS related to BPH. Ther Adv Urol 2019 Oct 11;11:1756287219879533.
- Geavlete P, Multescu R, Geavlete B: Serenoa repens extract in the treatment of benign prostatic hyperplasia. Ther Adv Urol 2011; 3: 193-198.
- Mirone V, et al: Current benign prostatic hyperplasia treatment: impact on sexual function and management of related sexual adverse events. Int J Clin Pract 2011; 65: 1005-1013.
- Nickel JC, Sander S, Moon TD: A meta-analysis of the vascular-related safety profile and efficacy of alpha-adrenergic blockers for symptoms related to benign prostatic hyperplasia. Int J Clin Pract 2008; 62: 1547-1559.
- Logan IC, Witham MD: Efficacy of treatments for orthostatic hypotension: a systematic review. Ageing 2012; 41: 587-594.
- Man in’t Veld AJ: Symptomatic BPH and hypertension: does comorbidity affect quality of life? Eur Urol 1998; 34(Suppl. 2): 29-36.
- Schimke L, Schimke J: Urological implications of falls in the elderly: lower urinary tract symptoms and alpha-blocker medications. Urol Nurs 2014; 34: 223-229.
- Drug Information, www.swissmedicinfo.ch,(last accessed Sept. 15, 2023).
- Metzker H, Kieser M, Hölscher U: Efficacy of a Sabal-Urtica combination preparation in the treatment of benign prostatic hyperplasia (BPH). Urologist B 1996; 36: 292-300.
- Sökeland J, Albrecht J: Combination of sabal and urtica extract vs. finasteride in BPH (stad. I to II according to Alken). Comparison of therapeutic efficacy in a one-year double-blind study. Urologist A 1997; 36: 327-333.
- Lopatkin N, et al: Long-term efficacy and safety of a combination of sabal and urtica extract for lower urinary tract symptoms – a placebo-controlled, double-blind, multicenter trial. World J Urol 2005; 23: 139-146.
- Engelmann U, et al.: Efficacy and safety of a combination of sabal and urtica extract in lower urinary tract symptoms. A randomized, double-blind study versus tamsulosin. Drug Research 2006; 56: 222-229.
- McConnell J, et al. (Eds): Male lower urinary tract dysfunction. Evaluation and manage-ment. In: Proceedings of the6th International Consultation on New Developments in Prostate Cancer and Prostate Diseases, Paris, France, 24-27 June 2005. Health Publications, 2006.
- Sivkov A, et al: Longterm efficacy and safety of a combination of sabal and urtica extracts in LUTS – a placebo-controlled, double-blind, multicenter trial. Urologist A 2001; 40(Suppl. 1): S19.
- Barry MJ, et al: Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association symptom index and the benign prostatic hyperplasia impact index is perceptible to patients? J Urol 1995; 154: 1770-1774.
- Lopatkin et al: Efficacy and safety of a combination of Sabal and Urtica extract in lower urinary tract symptoms – long-term follow-up of a placebo-controlled, double-blind, multicenter trial. Int Urol Nephrol 2007; 39: 1137-1146.
- Madersbacher S, et al: Association between PRO 160/120 prescriptions and incidence of benign prostatic hyperplasia complications in Germany: a retrospective cohort study. Postgrad Med 2023; 135(2): 149-154.
- Koch E: Extracts from fruits of saw palmetto (Sabal serrulata) and roots of stinging nettle (Urtica dioica): viable alternatives in the medical treatment of benign prostatic hyperplasia and associated lower urinary tracts symptoms. Planta Med 2001; 67(6): 489-500.
- Casarosa C, et al: Lack of effects of a lyposterolic extract of Serenoa repens on plasma levels of testos-terone, follicle-stimulating hormone, and luteinizing hormone. Clin Ther 1988; 10: 585-588.
- Düker EM, Kopanski L, Schweikert HU: Inhibition of 5α-reductase activity by extracts from Sabal serrulata. Planta Med 1989; 55: 587.
- Goepel M, et al: Saw palmetto extracts potently and noncompetitively inhibit human 1-adrenoceptors in vitro. Prostate 1999; 38: 208-215.
- 23 Abe M, et al: Pharmacologically relevant receptor binding characteristics and 5α-reductase inhibitory activ-ity of free fatty acids contained in saw palmetto extract. Biol Pharm Bull 2009; 32: 646-650.
- Suzuki M, et al: Muscarinic and alpha 1-adrenergic receptor binding characteristics of saw palmetto ex-tract in rat lower urinary tract. Urology 2007; 69: 1216-1220.
- Hirano T, Homma M, Oka K: Effects of stinging nettle root extracts and their steroidal components on the Na+, K+-ATPase of the benign prostatic hyperplasia. Planta Med 1994; 60: 30-33.
- Popa G, Hägele-Kaddour H, Walther C: Symptomatic efficacy of a Sabal-Urtica combination preparation in the therapy of benign prostatic syndrome. Results of a double-blind, placebo-controlled study. MMW Fortschr Med 2005; 147(Originals III): 103-108.
- Koch E, Biber A: Pharmacological effects of sabal and urtica extracts as a basis for rational drug therapy of benign prostatic hyperplasia. Urologist B 1994; 34: 90-95.
- Lichius JJ, et al: Antiproliferative effect of a polysaccharide fraction of a 20% meth-anolic extract of stinging nettle roots upon epithelial cells of the human prostate (LNCaP). Pharmacy 1999; 54: 768-771.
- Sökeland J: Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: analysis of prostate volume and therapeutic outcome. BJU Int 2000; 86: 439-442.
- Sökeland J, Schläfke S: Long-term effects of PRO 160/120 in patients with BPH. Uro-News Therapy Report Current 2007; 164: 2-3.