Aplastic anemia is associated with bone marrow hypocellularity and pancytopenia. In patients with immune-mediated severe or very severe aplastic anemia for whom bone marrow transplantation is not an option, the addition of an oral thrombopoietin receptor agonist to standard immunosuppressive therapy has been shown to be beneficial.
In aplastic anemia, there is a complete lack of erythrocytes, leukocytes and thrombocytes due to bone marrow insufficiency. This results in a hypo- to aplastic bone marrow, as CD34-positive hematopoietic stem cells are displaced by fat marrow. Depending on the cause, different pathophysiological mechanisms are involved, explained Dr. Neal S. Young from the National Heart, Lung and Blood Institute of the National Institutes of Health, Bethesda (USA) at the Annual Meeting of the European Hematology Association (EHA) [1]. The three most important pathomechanisms are
- Chemical or physical damage, e.g. due to chemotherapy or radiotherapy
- constitutional defect in genes that are important for maintaining cell integrity and immune regulation
- immune-mediated damage to the hematopoietic cells.
The latter occurs most frequently and is also referred to as “idiopathic aplastic anemia”. It is assumed to be an autoimmune reaction caused by activated T lymphocytes against blood stem and progenitor cells. The blood count shows normocytic anemia (MCV normal) with reticulocytopenia, pronounced thrombocytopenia and leukopenia with neutropenia. There are few or no precursors in the bone marrow aspirate and the bone marrow biopsy is characterized by greatly reduced cellularity with proliferation of fatty marrow. The clinical picture is characterized by anemia (pallor, fatigue), neutropenia (infection) and thrombocytopenia (bleeding) [2].
Treatment options at a glance
Hematopoietic stem cell transplantation is a potentially curative treatment option. This is the treatment of choice, particularly for younger patients with a suitable donor. For this reason, siblings are examined for compatibility of the human leukocyte antigen as soon as the diagnosis is made.
For patients who are not suitable for a transplant or who do not have a donor, immunosuppressive treatment with equine antithymocyte globulin (ATG) in combination with ciclosporin is available as an alternative. This treatment option is effective in the majority of patients, but not in all, according to Dr. Young [1]. Around two thirds of patients respond to it [3]. For many years, various methods have been tried unsuccessfully to improve the results of standard immunosuppressive therapy (IST). Finally, a breakthrough was achieved when an open, non-randomized phase I/II study showed that the addition of Eltrombopag to ATG plus ciclosporin resulted in considerable response rates in treatment-naive patients with severe or very severe aplastic anemia [4].
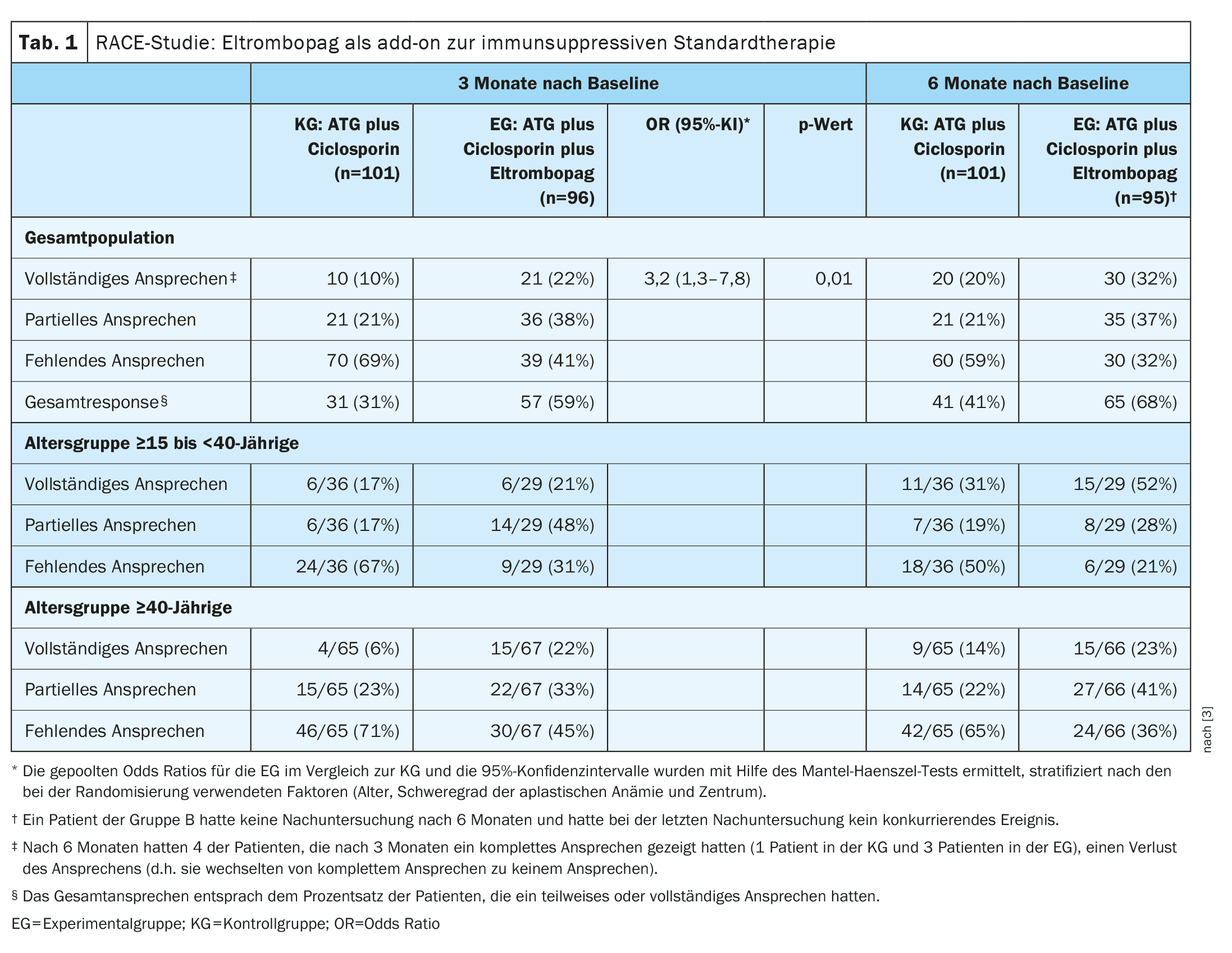
| The primary endpoint of the study was a complete hematologic response at three months, defined as a hemoglobin level above 10 g per dL, an absolute neutrophil count above 1000 per mm3 and a platelet count >100 000 per mm3 in patients who had not received transfusions. The criteria for a partial response were independence from transfusions (both red cells and platelets), with a blood group that did not meet the criteria for severe aplastic anemia but was insufficient for a complete response. |
Eltrombopag boosts hematopoiesis to stem cell level
This oral thrombopoietin receptor agonist acts on the control of blood stem cell and platelet formation. The drug activates thrombopoietin, which controls the formation of thrombocytes and blood (hematopoiesis). In the randomized-controlled phase III study RACE (Randomized, Multicenter Trial Comparing Horse ATG plus Cyclosporine with or without Eltrombopag as First-Line), IST alone (ATG plus Ciclosporin) was compared with IST plus Eltrombopag as first-line therapy in patients with severe or very severe aplastic anemia [3]. The median time to first response and to complete response was found to be shorter in the study arm with IST plus eltrombopag (experimental group, EG) compared to the study arm in which IST alone was prescribed (control group, KG). These shorter response times explain why EG was previously independent of red blood cell and platelet transfusions.
- The KG patients received IST consisting of ATG (40 mg per kg bw daily for four consecutive days) plus oral ciclosporin (5 mg per kg bw daily from the first day for at least 12 months). Ciclosporin was then reduced over the following 12 months and discontinued after 24 months.
- The patients in the EG received the experimental therapy consisting of IST plus eltrombopag (150 mg daily from day 14). The demographic and clinical characteristics of the participants in both groups were comparable. The median follow-up period was 24 months.
- The percentage of patients who responded completely to treatment after three months was 10% in the CG and 22% in the EC (pooled odds ratio [OR], 3.2; 95% CI: 1.3-7.8; p=0.01, Tab. 1).
- The overall response rate at 3 months was lower in the control group (31%) than in the EC (59%) and the median time to first response was 8.8 months in the control group and 3.0 months in the EC. After 12 months, the complete response rate was 33% in the control group and 52% in the EC. The median time to first response was 3.0 months in the eltrombopag arm and 8.8 months in the EC arm.
The incidence of severe adverse effects was comparable in both groups; treatment-associated treatment interruptions were rare. Somatic mutations were detected in 31% in the EG and 29% in the KG before the start of the study. These percentages increased to 55% in the EG and 66% in the KG by month 6 after baseline, with no effect on the hematologic response and 2-year survival rates (90% in the EG vs. 85% in the KG).
Haploidentical bone marrow transplants are also promising in order to expand access to hematopoietic stem cell transplantation, the speaker mentioned [1,5].
Congress: EHA2024
Literature:
- “Immune aplastic anemia: Update on Pathophysiology and New Treatments”, Dr. Neal S. Young, EHA2024, Madrid, 13.6.-16.6.2024.
- Lauten M, Erlacher M, Knöfler R: Hematology. Pediatrics 2019: 541-570.
- Peffault de Latour R, et al: Eltrombopag Added to Immunosuppression in Severe Aplastic Anemia. N Engl J Med 2022; 386(1): 11-23.
- Townsley DM, et al: Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med 2017; 376: 1540-1550.
- DeZern A, et al: Alternative donor BMT with post-transplant cyclophosphamide as initial therapy for acquired severe aplastic anemia [published online ahead of print, 2023 Apr 21]. Blood 2023; blood.2023020435. doi:10.1182/blood.2023020435.
HAUSARZT PRAXIS 2024; 19(11): 34–35 (published on 25.11.24, ahead of print)
InFo ONKOLOGIE & HÄMATOLOGIE 2024; 12(6): 26–27