The standard of care for newly diagnosed glioblastoma is resection as completely as possible. This is followed by temozolomide-based radiochemotherapy. Among the newer therapeutic approaches is the use of tumor therapy fields.
Glioblastomas are the most common malignant primary brain tumors in adults and can basically occur at any age. The incidence increases with age and the median age of onset is 64 years. Men are affected slightly more often than women [1]. The median survival of patients with newly diagnosed glioblastoma is only about 16 months, even in clinical trials, and is much shorter in population-based studies.
In contrast to the only relatively minor therapeutic advances, the understanding of the molecular biology of glioblastoma has expanded considerably over the past decade. According to the WHO classification revised in 2016, glioblastomas that arise de novo do not have a mutation in either of the isocitrate dehydrogenase (IDH) genes. This group accounts for about 90% of all glioblastomas. In contrast, the much rarer glioblastomas arising from a low-grade tumor usually have an IDH mutation and have a better prognosis. Accordingly, these tumors are reported separately in the new WHO classification [2]. O6-methylguanine DNA methyltransferase (MGMT) has emerged as an important biomarker. It is a DNA repair protein that reverses alkylations at the O6 position of guanine. Methylation of the MGMT promoter is found in approximately one-third of glioblastomas. Result of methylation is absent or decreased MGMT expression, leading to sensitivity of cells to alkylating chemotherapy.
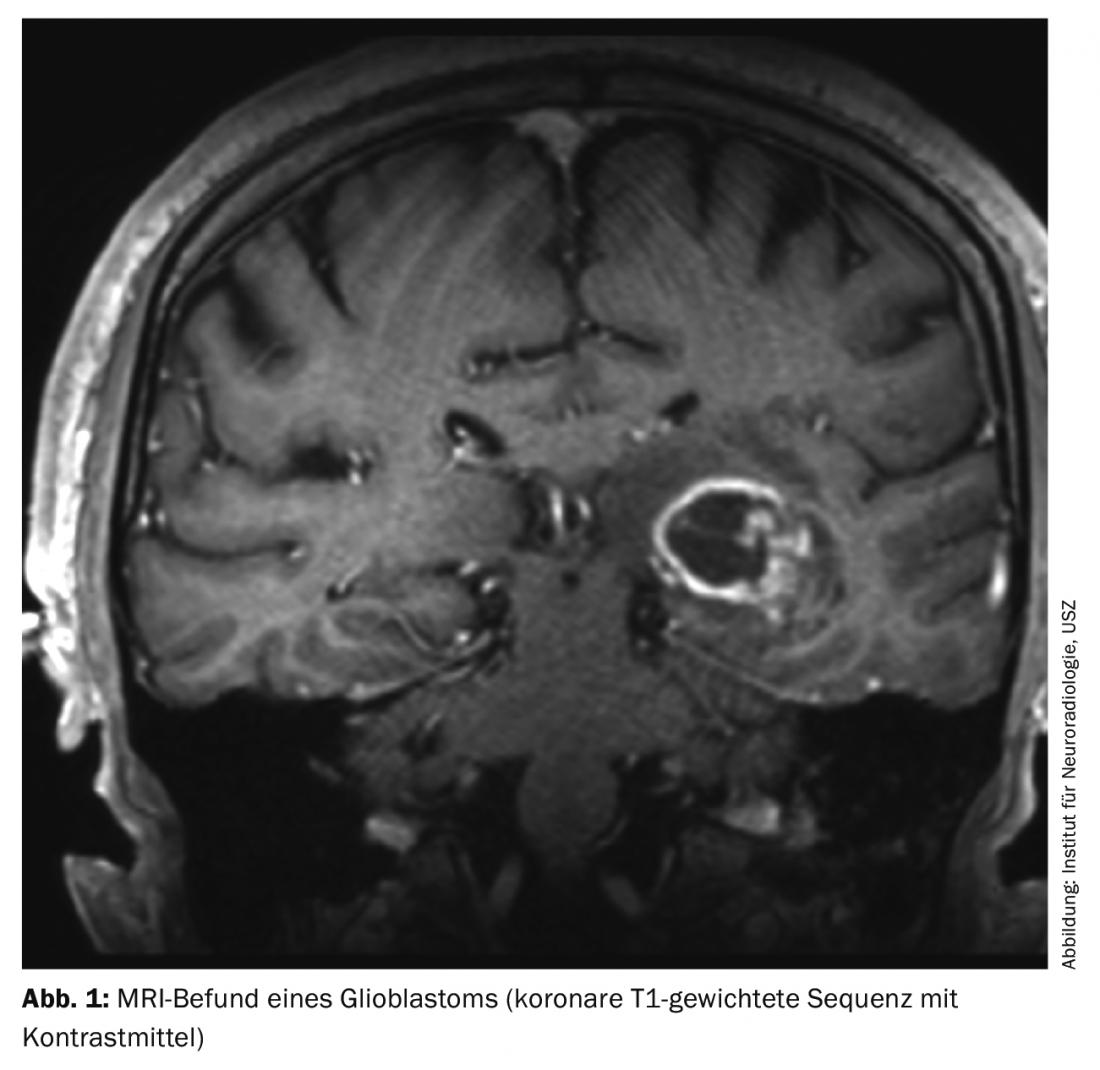
Glioblastomas manifest themselves by different clinical symptoms, which depend primarily on the location of the tumor in the brain. In addition to personality changes, headache, nausea and vomiting in the sense of intracranial pressure symptoms or focal neurological deficits, an epileptic seizure often leads to a diagnostic workup. Cranial MRI typically shows a marginally contrast-enhancing lesion with central necrosis (Fig. 1). If a glioblastoma or other brain tumor is suspected on imaging, histologic confirmation of the diagnosis must usually be sought.
Therapy
The goal of the surgical procedure, in addition to obtaining tissue, is to obtain the maximum resection that can be performed without causing new neurologic deficits. Tumors for which complete resection is not possible because of localization in the brain should be biopsied only. The same applies to patients in reduced general condition who may not be able to withstand the stresses of extensive surgery. The use of 5-aminolevulinic acid and intraoperative imaging can improve the extent of resection. This leads to a prolongation of progression-free survival [3,4].
Since 2005, radiotherapy with concomitant temozolomide followed by maintenance temozolomide for up to six cycles (TMZ/RT -> TMZ) has been the standard therapy for patients with newly diagnosed glioblastoma. Radiochemotherapy is superior to radiotherapy alone in this regard [5]. Temozolomide is an alkylane that can be administered orally and has an overall favorable side effect profile. The most common side effects include fatigue and nausea, as well as myelosuppression, some of which require dose adjustment.
Several attempts to add other agents to temozolomide-based radiochemotherapy have not resulted in prolonged survival. This applies in particular to the antibody bevacizumab, which is directed against the vascular endothelial growth factor (VEGF). The addition of bevacizumab to standard therapy resulted in prolonged progression-free survival in patients with newly diagnosed glioblastoma, but not in prolonged overall survival [6,7]. The peptide vaccine rindopepimut, which targets the mutant vIII variant of the “epidermal growth factor receptor” (EGFRvIII), was also investigated in a randomized trial in patients with EGFRvIII-positive, newly diagnosed glioblastoma. However, no survival benefit was found compared with placebo vaccine [8].
In contrast to the aforementioned drugs, a new treatment approach using so-called tumor treating fields has been shown to prolong survival [9]. Here, an electric field is generated via electrodes to be applied to the skull, which should lead to an inhibition of tumor cell proliferation. Treatment with tumor therapy fields is in addition to maintenance therapy with temozolomide. The device should ideally be worn for at least 18 hours a day. The most common side effects include skin irritation from the adhesive electrodes. How many patients will opt for such treatment in addition to chemotherapy in the future remains to be seen. In addition, the issue of reimbursement by insurance carriers is currently unclear in most countries.
The therapy of elderly glioblastoma patients presents a particular challenge. In the meantime, a randomized trial demonstrated that the combination of radiotherapy and temozolomide chemotherapy was also superior to radiotherapy alone in these patients. However, temozolomide leads to a relevant survival benefit only in patients with an MGMT-methylated tumor [10]. Accordingly, radiation therapy alone may be considered, particularly in patients older than 70 years with MGMT-unmethylated tumors. Patients with MGMT-methylated tumor in whom combined radiochemotherapy is not indicated, for example, because of reduced general condition, may receive monotherapy with temozolomide [11]. Especially in elderly patients in reduced general condition, a palliative-supportive therapy concept should be evaluated at an early stage due to the very unfavorable prognosis.
Outlook
Currently, the focus of scientific interest is mainly on immunotherapeutic treatment approaches [12]. In addition to advanced vaccination concepts, inhibitors of the programmed cell death (PD)-1 pathway are being investigated in particular. The PD1 inhibitor nivolumab is currently being used in two randomized phase III trials. The Checkmate 498 trial is comparing the combination of radiotherapy and nivolumab to standard therapy (TMZ/RT -> TMZ) in patients with MGMT-unmethylated glioblastoma. The parallel-recruiting Checkmate 548 trial is comparing the addition of nivolumab or placebo to TMZ/RT -> TMZ in patients with newly diagnosed glioblastoma in whom the tumor has methylation of the MGMT promoter. The results of these and other studies will show whether immunotherapeutic approaches can contribute to a better prognosis in glioblastoma patients.
Take-Home Messages
- Standard therapy for newly diagnosed glioblastoma consists of resection as complete as possible, followed by temozolomide-based radiochemotherapy.
- Adding bevacizumab to standard therapy does not prolong survival in glioblastoma patients.
- A phase III study, which was evaluated as positive, showed an extension of survival by several months through the use of tumor therapy fields.
- Studies currently underway are specifically investigating various immunotherapeutic treatment approaches.
Literature:
- Ostrom QT, et al: CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol 2016; 18(Suppl 5): v1-v75.
- Louis DN, et al: The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016; 131(6): 803-820.
- Stummer W, et al: Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006; 7(5): 392-401.
- Senft C, et al: Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol 2011; 12(11): 997-1003.
- Stupp R, et al: Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352(10): 987-996.
- Chinot OL, et al: Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014; 370(8): 709-722.
- Gilbert MR, et al: A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med 2014; 370(8): 699-708.
- Weller M, et al: Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol 2017; 18(10): 1373-1385.
- Stupp R, et al: Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA 2015; 314(23): 2535-2543.
- Perry JR, et al: Short-course radiation plus temozolomide in elderly patients with glioblastoma. N Engl J Med 2017; 376(11): 1027-1037.
- Weller M, et al: European Association for Neuro-Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncol 2017; 18(6): e315-e329.
- Weiss T, Weller M, Roth P: Immunotherapy for glioblastoma: concepts and challenges. Curr Opin Neurol 2015; 28(6): 639-646.
InFo ONCOLOGY & HEMATOLOGY 2017; 5(6): 16-18.