It is common for the diagnosis of PPCM to be made only in cardiogenic shock because of rapid exacerbation of heart failure or misinterpretation of symptoms as pregnancy-related or postpartum symptoms. According to a recent study, bromocriptine appears to contribute to cardiac function recovery.
Symptomatology and diagnostics
Cardinal symptoms of PPCM are progressive dyspnea and fatigue. Often, the condition is recognized late, as it is assumed to be pregnancy-related shortness of breath or common fatigue symptoms after delivery. Cough is not infrequently misinterpreted as a bronchopulmonary infection. It is common for PPCM to be diagnosed only in cardiogenic shock because of rapid exacerbation of heart failure or previous misinterpretation of symptoms.
It is difficult to differentiate the physiologic changes at the end of pregnancy associated with sinus tachycardia, shortness of breath, as well as edema from PPCM. Therefore, the awareness of all physicians involved must be raised for this clinical picture. In Germany, Denmark and the USA, very severe disease courses are described in about 15% of cases and mortality is 2-4% [1–3]. Fatal courses are much more common in South Africa and Turkey; mortality of 15-24% has been reported in these countries [4,5].
If heart failure-related dyspnea is suspected, the heart failure markers BNP or NT-proBNP and the highly sensitive cardiac enzyme troponin T or I should be determined in serum. If all values are within the normal range, a relevant cardiac insufficiency is practically excluded. If the values are elevated, further clarification is required. Depending on the symptoms, this can be done on an outpatient basis by a cardiologist or in the clinic. Echocardiography must be performed as the most important diagnostic procedure. When a reduced ejection fraction <45% is first demonstrated and other causes of impaired cardiac function are excluded, the diagnosis of PPCM is established. Other differential diagnoses such as myocardial infarction or pulmonary artery embolism must also be considered.
Definition and epidemiology
PPCM is defined as idiopathic systolic heart failure with a reduced ejection fraction <45% occurring in the last weeks of pregnancy to several months after delivery [6]. PPCM is a disease in its own right and not, as is often assumed, a worsening of a preexisting cardiomyopathy of other etiologies. Cardiac disease such as congenital heart defect, familial dilated cardiomyopathy, or cardiac injury after prior chemotherapy and/or thoracic radiation should be excluded. This is not always possible due to missing preliminary findings. However, almost all patients report well-being and good exercise tolerance before and often during pregnancy. Some had unremarkable echocardiography as part of a previous routine examination, for example, to evaluate for palpitations or arterial hypertension that occurred during pregnancy. In the presence of preexisting cardiomyopathy, heart failure symptoms usually begin in the 1. until 2nd trimester. The majority of PPCM patients do not become symptomatic until under or after delivery; at this time, although the circulatory load increases in the short term because the volume of distribution of maternal blood is reduced acutely in the now absent placental circulation, it decreases rapidly compared with late pregnancy.
It is difficult to separate PPCM from myocarditis. In a study of 1230 patients with cardiomyopathy of unclear etiology, these could be distinguished from each other on the basis of endomyocardial biopsies [7]. Further studies report that virus-positive findings also occur in healthy individuals [8]. Interestingly, many patients in German registries show only moderately or not elevated C-reactive protein (CRP) levels [1]. In contrast, autoimmune responses likely play an important role in the pathogenesis of PPCM [9]. Myocardial biopsy or cardiac catheterization is usually not necessary in typical PPCM.
The incidence of PPCM varies by region. In Nigeria, PPCM appears to be very common in certain regions, with 1 per 100 pregnancies. Haiti at 1 per 299 and South Africa at 1 per 1000 pregnancies are also hotspots [10]. In the United States, an increased incidence of 1 per 1149-3189 has been reported over the past several decades. In Germany, we assume 1 disease per 1000-1500 births.
Pathophysiology
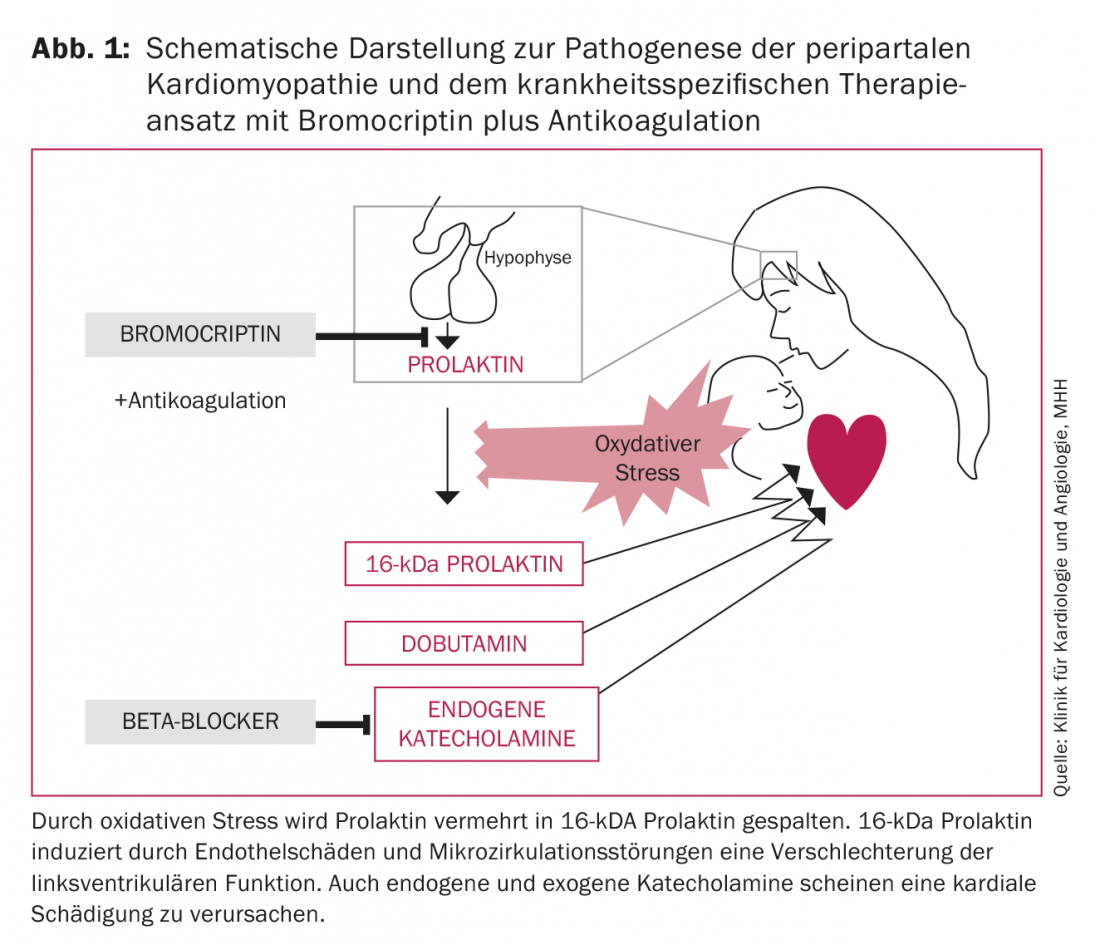
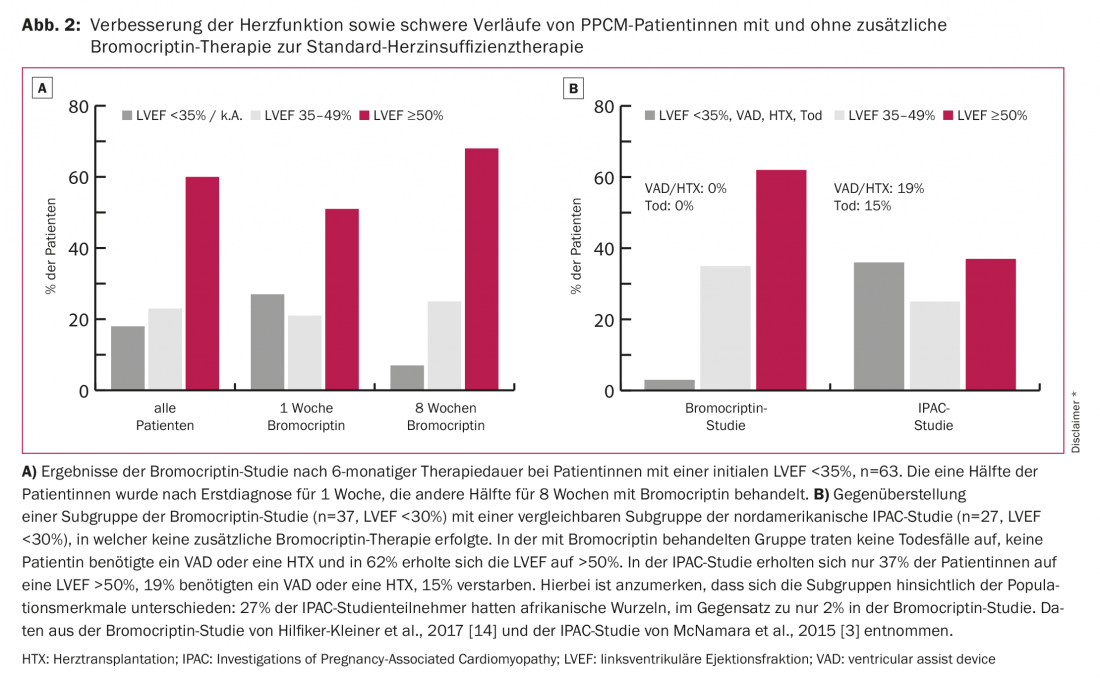
The triggers of PPCM are not yet adequately understood. Currently, the full-blown PPCM is thought to have a multifactorial etiology. Diverse etiologies such as inflammation, viral or bacterial infection, autoimmune disease, genetic features, and oxidative stress are discussed. The latter observation in particular is the first specific therapeutic approach in addition to heart failure therapy. Thus, Hilfiker-Kleiner et al. show that oxidative stress increases the cleavage of prolactin to 16-kDa prolactin. This 16-kDa cleavage product attacks the blood vessels and especially the endothelium, thus inducing endothelial dysfunction and capillary degradation. In addition, it acts as an angiostatic, preventing blood vessel regeneration [11]. This results in endothelial damage, microcirculatory disturbances, and thus deterioration of left ventricular (LV) function (Fig. 1). Therapy with the dopamine agonist bromocriptine inhibits prolactin secretion, prevents the development of heart failure in a mouse model 100% [11] and significantly improves LV function in PPCM patients [1,4,12,13]. The most favorable disease outcomes published to date were seen in the recently published German randomized multicenter trial of bromocriptine therapy in PPCM patients [14]: no deaths occurred with bromocriptine therapy, no patients required heart transplantation or mechanical cardiac assist devices, and left ventricular systolic function recovered completely in 62% (Fig. 2).
*This figure has been translated and reprinted by permission of Oxford University Press on behalf of the ESC. OUP and the ESC are not responsible or in any way liable for the accuracy of the translation. The translator is solely responsible for the translation in this publication.
Therapy
Hemodynamically stable patients with clinical signs of cardiac decompensation, such as dyspnea and leg edema, are recompensed under inpatient conditions with loop diuretics and rapidly started on heart failure therapy consisting of ACE inhibitor/AT-1 receptor antagonist, beta blocker, and mineralocorticoid receptor antagonist (MRA). The preparations should be increased to the maximum tolerated dose of standard heart failure therapy. If up-dosing of beta-blockers is not possible because of hypotensive blood pressure values (<90 mmHg systolic), heart rate lowering with ivabradine is recommended because early heart rate lowering seems to be associated with improved LV function [15]. In addition to standard heart failure therapy, treatment with bromocriptine should be given at a dose that depends on the severity of the disease process (Fig. 3) , because initial bromocriptine therapy is likely to attenuate the extent of the disease [14]. Because of the possible prothrombogenic properties of bromocriptine, anticoagulation is mandatory, at least in thromboprophylaxis doses.
Hemodynamically and or respiratory compromised patients must be treated in the ICU. If the systolic blood pressure is >110 mmHg, vasodilators can also be used. In cases of hemodynamic instability and indication for catecholamine therapy, the implantation of a temporary circulatory support system, such as an implantable heart pump (Impella®), should be considered at an early stage; in cases of circulatory and pulmonary failure, veno-arterial extracorporeal membrane oxygenation (ECMO) should also be considered. This is the only way to relieve the heart while saving or even completely eliminating the use of catecholamines. The use of catecholamines and especially dobutamine appears to be associated with increased mortality and should be avoided whenever possible [16,17]. More beneficial is probably the inotropic levosimendan, which does not increase cardiac oxygen consumption and has shown promising results in a small study [18]. The course of the disease varies greatly between individuals.
If hemodynamic stabilization can be achieved via the above therapies, our experience shows that slow recovery is still possible weeks later. It is essential to diagnose and treat comorbidities such as in utero infections or mastitis. An interdisciplinary approach is required, especially for these critically ill patients. If weaning from the temporary percutaneous support system cannot be achieved after approximately 14 days despite these measures, permanent support systems such as a surgically implanted ventricular assist device (VAD) are used.
Listing for heart transplantation should occur if cardiac function has not improved after 6-12 months and explantation of the VAD does not appear feasible. We treat intensive care patients who are artificially ventilated or require mechanical circulatory support with up to 20 mg of bromocriptine per day for at least eight weeks, depending on prolactin levels, with gradual dose reduction according to clinical evolution and control of prolactin levels (Fig. 3).
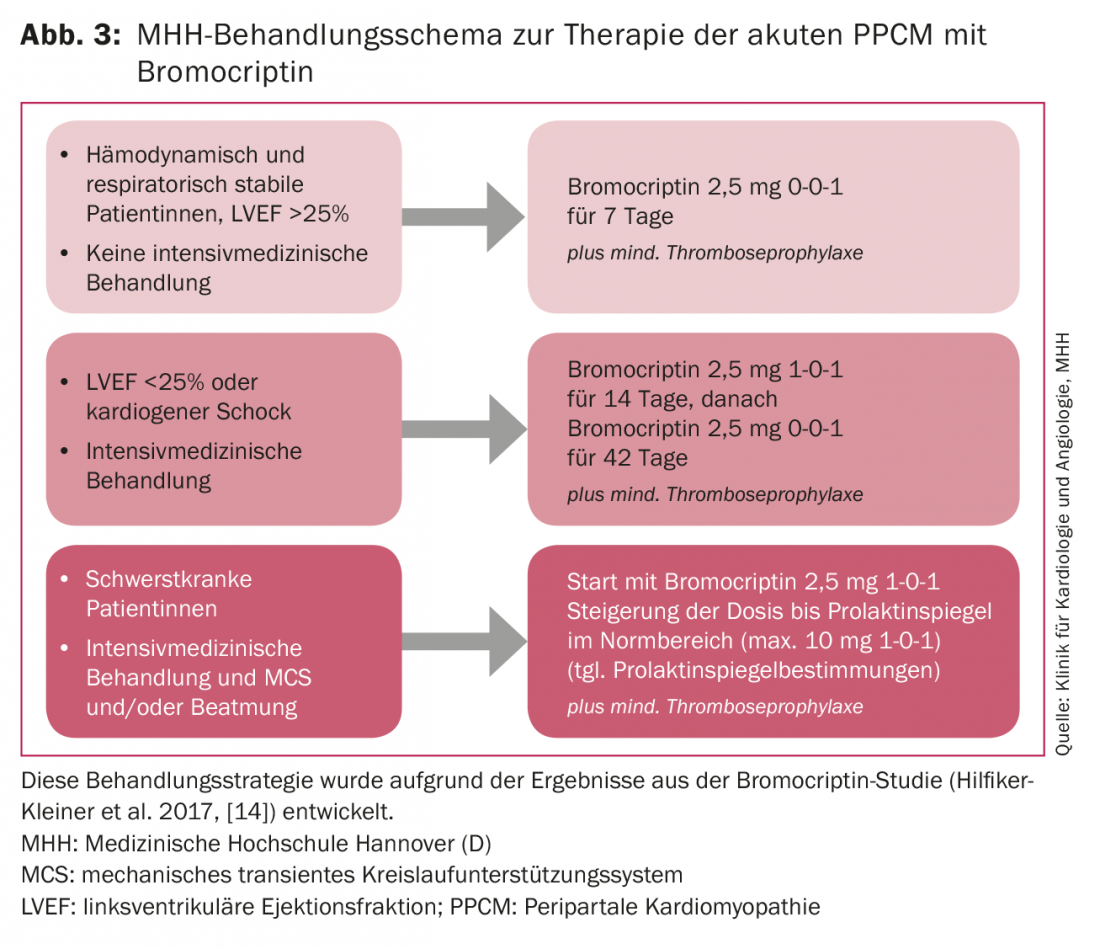
Circulatory support systems can lead to an increase in prolactin levels, so these must be closely monitored. Such dramatic courses are fortunately rare in Germany, and treatment planning always requires decisions on a case-by-case basis. Early transfer to an experienced center is necessary. A recommended treatment strategy for PPCM patients is summarized under the BOARD algorithm: Bromocriptine, Oral heart failure medication, Anticoagulation, VasoRelaxants and Diuretics [19].
Pregnant patients
Heart failure therapy with ACE inhibitors, AT-1 antagonists, and mineralocorticoid receptor antagonists is contraindicated during pregnancy. Beta-blockers can and should also be used in pregnancy for PPCM, and diuretics should be used with caution and only in symptomatic pulmonary venous congestion because of the risk for placental inferior perfusion.
Pregnancy can be continued in cardiopulmonary stable patients under close cardiologic and gynecologic care. Vaginal delivery is possible, but the delivery procedure must be decided individually as a team by the attending obstetricians and cardiologists. In order to reduce the heavy cardiac load in late pregnancy, primary sectio is often planned from the 36th week of pregnancy.
In hemodynamically unstable patients, delivery must be attempted immediately regardless of the age of the child, and if necessary. induce infantile lung maturity. In this case, primary sectio under combined spinal and epidural anesthesia is recommended [20].
After delivery, PPCM patients should be weaned on bromocriptine and continue bromocriptine intake, thereby inhibiting the formation of the 16-kDa prolactin cleavage product that contributes to the disease and avoiding the additional metabolic stress of breastfeeding for the mother. ACE inhibitors and mineralocorticoid receptor antagonists (MRAs) pass into breast milk. Therefore, optimal therapy of heart failure is possible only by weaning the child.
Aftercare
Heart failure therapy should follow the guidelines of the European Society of Cardiology [21]. PPCM, unlike other cardiomyopathies, has a high proportion of remissions. Meanwhile, >90% of patients in Germany improve and >60% show fully recovered LV function of ≥50%. Regeneration of LV function takes different lengths of time: in some patients, LV function returns to normal after only a few weeks; in others, the healing process may take several months or even years.
Therefore, patients with an LVEF <35% should initially be fitted with a wearable defibrillator vest to protect them from the increased risk of sudden cardiac death [22]. If necessary, this should be worn well beyond the 3-month period before a decision is made regarding an implantable defibrillator.
Even after complete recovery of LV function, we recommend continuing established heart failure therapy for at least six months. Only then, starting with MRA, can the substances be reduced and discontinued one at a time and under regular, echocardiographic monitoring [23]. There are always patients whose LV function deteriorates under reduced heart failure therapy, so that long-term therapy is necessary.
Re-pregnancy carries an increased risk of PPCM recurrence in all PPCM patients, especially if cardiac function has not recovered to an LVEF >50% [24]. This must be discussed with the patients and their partners. If a new pregnancy occurs – planned or unplanned – we recommend consultation at a PPCM specialty center. Existing heart failure medication should be changed if necessary to prevent potential side effects on the fetus. Pregnancy must be managed closely and interdisciplinarily, and delivery must be carefully planned. Immediate treatment with bromocriptine and heart failure medication after delivery usually suggests a favorable prognosis. Counseling should keep in mind that early abortion may not necessarily prevent the onset of PPCM. On the other hand, in many patients, subsequent pregnancy proceeds without serious complications even with impaired LV function.
Summary
PPCM is a heart failure that is often acute, but may also be subacute, occurring just before, during, or several months after birth. In addition to clinical symptoms, increased BNP or NT-proBNP levels and echocardiographic evidence of reduced LV function <45% are seminal. In order to avoid fatal courses, rapid diagnosis and initiation of therapy are indispensable. Long-term heart failure therapy is often required, and additional treatment with bromocriptine should be given even initially. Continued pregnancy, even after complete recovery of LV function, is associated with an increased risk of recurrence and should definitely be managed in expert centers.
Take-Home Messages
- Peripartum cardiomyopathy (PPCM) refers to heart failure occurring around the time of delivery in previously healthy women.
- Pre- or postpartum dyspnea should not be trivialized as pregnancy-associated or as a bronchopulmonary infection without further diagnostic testing.
- When peripartum heart failure is first diagnosed, it should be counseled and, if necessary, treated by an experienced center, because fatal courses recur.
- In addition to standard heart failure therapy, bromocriptine treatment appears to improve recovery of cardiac function.
Literature:
- Haghikia A, et al: Phenotyping and outcome on contemporary management in a German cohort of patients with peripartum cardiomyopathy. Basic Res Cardiol 2013; 108: 366.
- Ersbøll AS, et al: Peripartum cardiomyopathy in Denmark: a retrospective, population-based study of incidence, management and outcome. Eur J Heart Fail 2017 Jun 8. doi: 10.1002/ejhf.882. [Epub ahead of print].
- McNamara DM, et al: Clinical Outcomes for Peripartum Cardiomyopathy in North America. Results of the IPAC Study (Investigations of Pregnancy-Associated Cardiomyopathy). J Am Coll Cardiol 2015; 66: 905-914.
- Sliwa K, et al: Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: a proof-of-concept pilot study. Circulation 2010; 121(13): 1465-1473.
- Biteker M, et al: Delayed recovery in peripartum cardiomyopathy: an indication for long-term follow-up and sustained therapy. Eur J Heart Fail 2012; 14(8): 895-901.
- Sliwa K, et al: Current state of knowledge on aetiology, diagnosis, management, and therapy of peripartum cardiomyopathy: a position statement from the Heart Failure Association of the European Society of Cardiology Working Group on peripartum cardiomyopathy. Eur J Heart Fail 2010; 12: 767-778.
- Felker GM, et al: Underlying causes and long-term survivals in patients with initially unexplained cardiomyopathy. N Engl J Med 2000; 342(15): 1077-1084.
- Selle T, et al: Reviewing peripartum cardiomyopathy: current state of knowledge. Future Cardiol 2009; 5: 175-189.
- Haghikia A, et al: Evidence of autoantibodies against cardiac troponin I and sarcomeric myosin in peripartum cardiomyopathy. Basic Res Cardiol 2015; 110(6): 60.
- Hilfiker-Kleiner D, Sliwa K: Pathophysiology and epidemiology of peripartum cardiomyopathy. Nat Rev Cardiol 2014; 11(6): 364-370.
- Hilfiker-Kleiner D, et al: A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 2007; 128: 589-600.
- Jahns B, et al: Peripartum cardiomyopathy – a new treatment option by inhibition of prolactin secretion. Am J Obstet Gynecol 2008; 199(4): e5-6.
- Meyer GP, et al: Bromocriptine treatment associated with recovery from peripartum cardiomyopathy in siblings: two case reports 2010; 4: 80.
- Hilfiker-Kleiner D, et al: Bromocriptine for the treatment of peripartum cardiomyopathy: a multicentre randomized study. Eur Heart J 2017; 38(35): 2671-2679.
- Haghikia A, et al: Early ivabradine treatment in patients with acute peripartum cardiomyopathy: subanalysis of the German PPCM registry. Int J Cardiol 2016; 216: 165-167.
- Stapel B, et al: Low STAT3 expression sensitizes to toxic effects of β-adrenergic receptor stimulation in peripartum cardiomyopathy. Eur Heart Jour 2017; 36: 349-361.
- Bauersachs J, et al: Current management of patients with severe acute peripartum cardiomyopathy: practical guidance from the Heart Failure Association of the European Society of Cardiology Study Group on peripartum cardiomyopathy. Eur J Heart Fail 2016; 18(9): 1096-1105.
- Labbene I, et al: Decongestive effects of levosimendan in cardiogenic shock induced by postpartum cardiomyopathy. Anaesth Crit Care Pain Med 2017; 36(1): 39-42.
- Arrigo M, et al: Bromocriptine for the treatment of peripartum cardiomyopathy: welcome on BOARD. Eur Heart J 2017; 38(35): 2680-2682.
- European Society of Gynecology (ESG), Association for European Paediatric Cardiology (AEPC), German Society for Gender Medicine (DGesGM), et al: ESC Guidelines on the management of cardiovascular diseases during pregnancy. The Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart Jour 2011; 32: 3147-3197.
- Ponikowski P, et al.: 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Eur J Heart Fail 2016; 18: 891-975.
- Duncker D, et al: Risk for life-threatening arrhythmia in newly diagnosed peripartum cardiomyopathy with low ejection fraction: a German multi-center analysis. Clin Res Cardiol 2017; 106(8): 582-589.
- Hilfiker-Kleiner D, et al: Peripartum cardiomyopathy: current management and future perspectives. Eur J Heart Fail 2015; 36: 1090-1097.
- Hilfiker-Kleiner D, et al: Outcome of subsequent pregnancies in patients with a history of peripartum cardiomyopathy. Eur J Heart Fail 2017 Mar 27. doi: 10.1002/ejhf.808. [Epub ahead of print].
CARDIOVASC 2018; 17(1): 8-12