Many herbal preparations are characterized by a gentle effect with few side effects. The opinion is also often expressed that phytotherapy acts rather slowly and weaker compared to synthetic agents. The following article shows that there are herbal medicines that are equal to corresponding synthetic preparations.
The studies presented here [1,2] were published several years ago, but have not lost their validity. Sökeland and Albrecht compared the clinical efficacy of the herbal extract PRO 160/120 (box) with the established synthetic agent finasteride in a study published in 1997. Sökeland later did a subgroup evaluation with the results of this study, verifying the claim of Boyle et al. [3], the efficacy of therapeutic treatment of benign prostatic hyperplasia (BPH) depends on prostate volume. Only in patients with a prostate volume of ≥40 ml is it possible to achieve clinical efficacy with drug treatment, he said. In this subgroup analysis, results were re-evaluated separately for patients whose baseline prostate volume was >40 ml and those with a baseline volume of ≤40 ml.

Comparison PRO 160/120 with Finasteride
Sökeland and Albrecht [1] compared the efficacy of the herbal combination preparation PRO 160/120 with the established synthetic agent finasteride in patients suffering from Alken’s stage I-II BPH. At the time of publication of this study, Alken I, II, and III were the standard for grading the severity of BPH. This classification has since been superseded by the International Prostate Symptom Score (IPSS). Alkene I and II correspond to an IPSS of 8-19.
In the study by Sökeland and Albrecht, the change in maximum urinary flow was the primary outcome variable. Secondary outcome variables were defined as mean urine flow, total urine volume, micturition time, time course of urine flow increase, prostate volume, IPSS, and quality of life. The study recruited 543 men (50-88 years old), all of whom had BPH (Alken I or II) and had a maximum urinary flow of 20 ml/s. Urine volume had to be less than 150 ml/miction. The demographic and clinical values of the recruited subjects did not show significant differences.
Treatment
The treatment enrolled 516 subjects who were randomized into two groups after a washout period of two weeks. There were 261 patients in the finasteride group and 255 patients in the PRO 160/120 group. Subjects received either one capsule of PRO 160/120 and placebo twice daily or placebo and one capsule of 5 mg finasteride twice daily for 28 weeks.
Mean prostate volume differed insignificantly in the two treatment groups, being 44.0 ml for the finasteride group and 42.7 ml for the PRO 160/120- group.
Results
After 48 weeks of treatment, the volume in the Pro 160/120 group had hardly changed and averaged 42.2 ml. However, in the finasteride group, the mean volume decreased by 5.2 ml. No significant difference was found between the two treatment groups for the primary outcome variable, maximum urine flow (Table 1) . In the PRO 160/120 group, maximum urine flow increased by 1.9 ml over the course of the study. In the finasteride group, the increase was 2.7 ml. However, this difference was not significant (p=0.73).
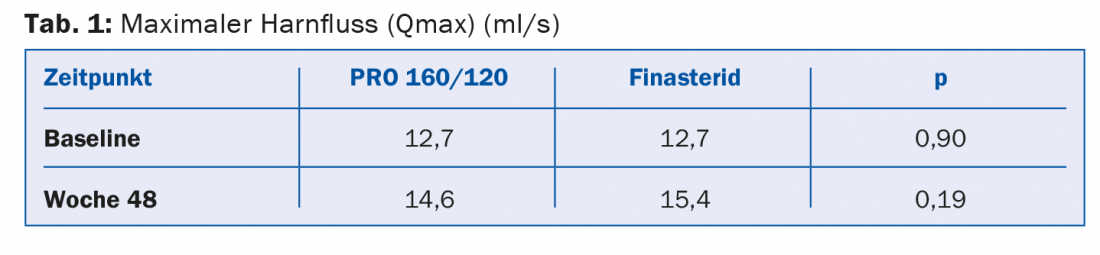
There were also no significant differences between the finasteride and PRO 160/ 120 groups in terms of change in IPSS (Table 2).
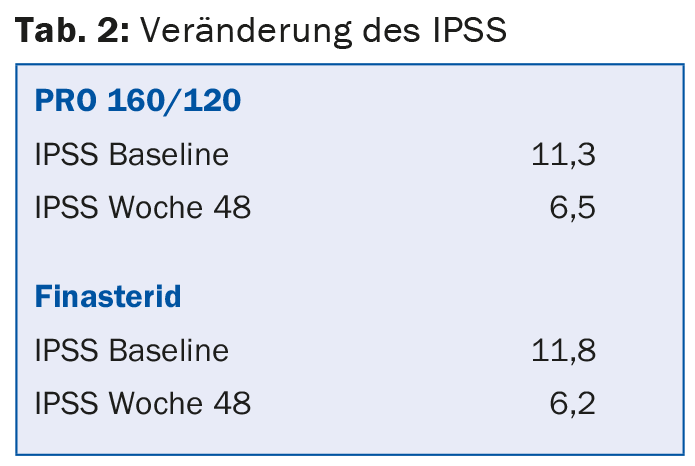
Maximum urine volume also showed little difference between the two treatment groups – the same was true for the other outcome variables mean urine flow, micturition time and quality of life.
On the other hand, significantly fewer side effects were documented in the PRO 160/120 group than in the finasteride group.
Discussion
The study by Sökeland and Albrecht documents the equivalence of the herbal extract PRO 160/120 in comparison with the synthetic substance finasteride. In terms of the primary outcome variable (maximum urinary flow) and all other important parameters, the two treatments were equivalent. The greater decrease in prostate volume in the finasteride group was not significant and had no clinical relevance.
Subgroup evaluation
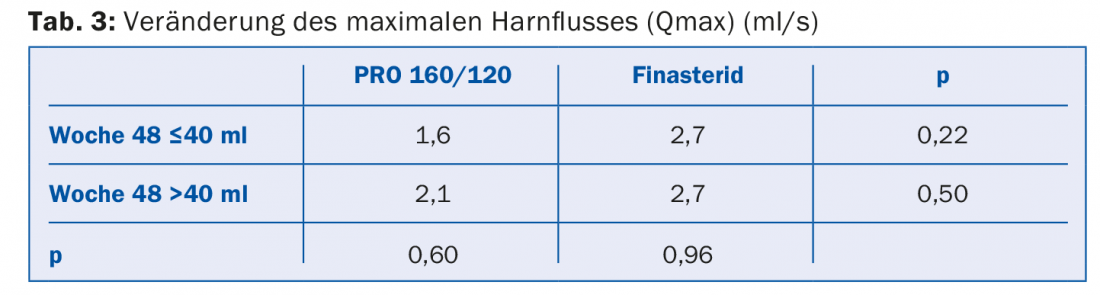
Sökeland [2], in his subgroup evaluation of the previous study [1], addressed the hypothesis that clinical efficacy could only be achieved with treatment if the prostate volume was ≥40 ml. For this purpose, he re-evaluated the results obtained by him and Albrecht, but separately for patients with a prostate volume at baseline ≤40 ml or >40 ml. For the primary outcome variable, maximum urinary flow, he obtained the values shown in Table 3. There were no significant differences between the different groups. The prostate volume and IPSS showed the same result.
Summary
The study by Sökeland and Albrecht [1] and the subsequent subgroup evaluation by Sökeland [2] document the equivalence of the herbal extract PRO 160/120 compared with finasteride with respect to the BPH-relevant outcome variables maximum urinary flow, total urinary volume, prostate volume, and IPSS in patients with BPH with an IPSS score of 8-19. None of these measurements showed a significant difference between the two treatment groups. PRO 160/120, however, had a better safety profile.
The subgroup evaluation also refuted the hypothesis of Boyle et al. that clinical efficacy can be achieved in BPH with drug treatment only when the baseline prostate volume is ≥40 ml.
Literature:
- Sökeland J, Albrecht L: Combination of sabal and urtica extract vs. finasteride in BPH (stad. I to II according to Alken). Urologist (A) 1997; 36: 327-333.
- Sökeland J: Combined sabal and urtica extract compared with finasteride in men with benign prostatic hyperplasia: analysis of prostate volume and therapeutic out-come. BJU International 2000; 86: 439-442.
- Boyle P, Gould AL, Roehrborn CG: Prostate volume predicts outcome of treatment of BPH with finasteride: meta-analysis of randomised clinical trials. Urology 1996; 48: 398-405.
HAUSARZT PRAXIS 2015; 10(11):2-3











