The introduction of new drugs has steadily improved the prognosis of patients with multiple myeloma over the past years. Diagnostics have also been expanded, in particular by cytogenetic analyses, which allow a more precise risk stratification. Nevertheless, the 5-year survival rate in stage III is only 40%, so there is still room for innovation.
As a B-cell lymphoma with monoclonal proliferation of plasma cells in the bone marrow, multiple myeloma (MM) is an extremely heterogeneous disease. While approximately one-quarter of affected individuals are asymptomatic at the time of diagnosis, acute courses with rapid bone destruction, marked renal dysfunction, fatigue, hypercalcemia, and propensity to infection also exist [1]. As the population ages, the number of new cases increases, with most cases occurring between the ages of 70 and 80. Multiple myeloma is still a rare disease, with an incidence of about 6 cases per 100,000 population in men and 4 cases per 100,000 population in women, but a one-third increase in the number of cases can be expected by 2040 due to the shift in age structures alone [2,3]. The requirements for efficient management with effective therapeutic options and target-oriented diagnostics will therefore continue to increase in the future.
View of the pathophysiology
In multiple myeloma, the malignant plasma cells produce complete or incomplete monoclonal immunoglobulins. These can be detected as clonally increased light chains in serum and urine and also bear the name “paraprotein”. In serum protein electrophoresis, they appear in the form of the famous “M gradient”. The high concentration of these immunoglobulins can cause various symptoms, such as AL amyloidosis due to the deposition of misfolded proteins. In addition, hyperviscosity syndrome may occur and, much more commonly, renal function deterioration. This is because the increased light chains are filtered glomerularly and often cannot be completely reabsorbed in the tubules. This leads to an excretion in the urine called “Bence-Jones proteinuria”. In addition, the paraproteins accumulate in the glomeruli and precipitate in the distal tubule by binding to the Tamm-Horsfall protein produced by the tubular epithelial cells. Findings: Glomerulosclerosis and cast nephropathy [4].
Other symptoms of multiple myeloma arise primarily from displacement of normal hematopoiesis and destruction of bone. These mechanisms lead to bone pain, pathological fractures, hypercalcemia, anemia, and susceptibility to infection, among others [1]. Often the complaints of those affected are non-specific, so that it is not uncommon for several months to pass before a correct diagnosis is made.
The genetics of multiple myeloma are as varied as the clinical presentation. Trisomies are found in about 40% of patients. Translocations involving the immunoglobulin heavy chain (IgH) locus on chromosome 14q32 are also common. Together with the trisomies, these belong to the primary genetic alterations and can already be detected in preliminary stages of the disease [5]. As the disease progresses, secondary genetic aberrations such as del(1p) or RAS mutations are added, which shape the clinic, treatment response and prognosis [3,6].
The bottom line is that the etiology of multiple myeloma is unclear. Familial clustering is rare and risk factors remain unclear to date [3]. The disease usually develops from a monoclonal gammopathy of uncertain significance (MGUS) [7]. If this precursor is present, the risk of progression to multiple myeloma or other lymphoproliferative disease requiring treatment is approximately 1% annually [8]. However, early detection of precursors has not been established, as it has not yet led to a significant improvement in prognosis [3].
Diagnostics in transition
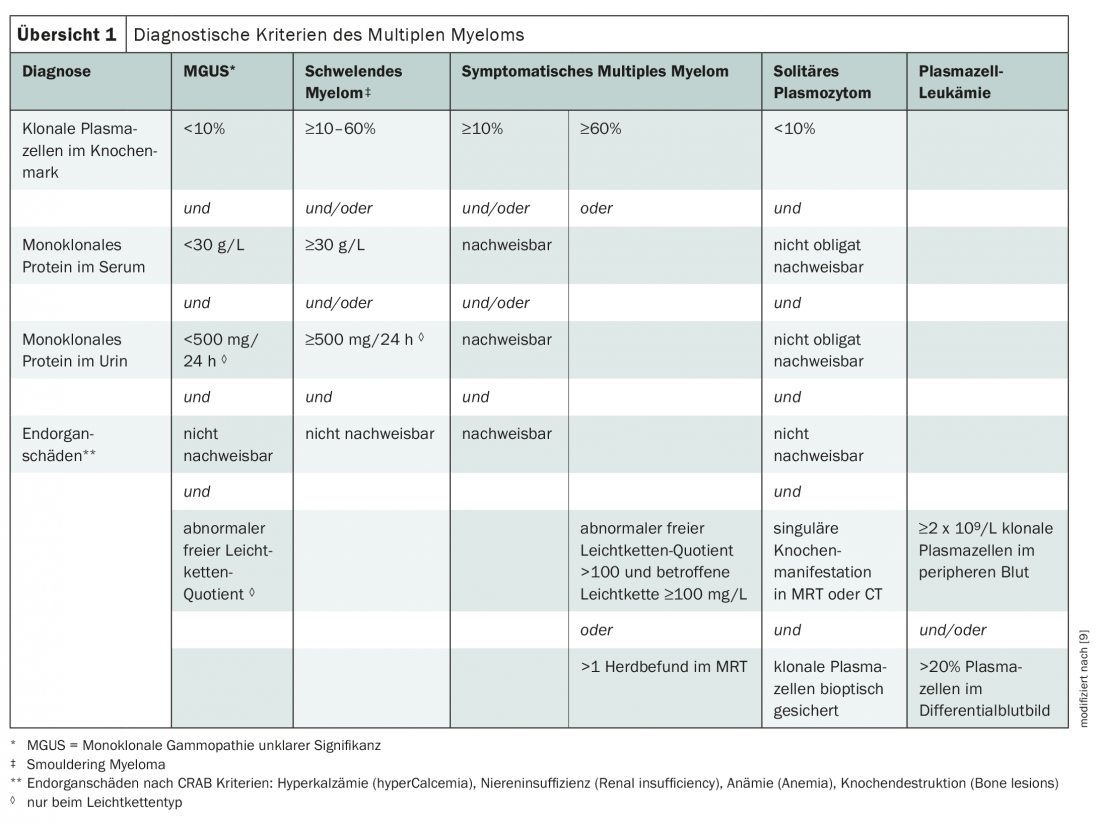
Currently, the International Myeloma Working Group criteria are primarily used to diagnose multiple myeloma (overview 1) [3,9]. The disease is classified by paraprotein type, with IgG and IgA myelomas being the most common. If only incomplete immunoglobulins, i.e. light chains, are formed, one speaks of “light chain myelomas”. These account for about one-fifth of cases [3].
In addition to history, physical examination, blood count, and laboratory, a low-dose whole-body CT scan and bone marrow aspiration are also part of the initial diagnostic workup. Imaging is used to detect osteolysis and osteopenia [3]. If necessary, this can be extended by an MRI scan for more precise differentiation of bone foci and diagnosis of extramedullary manifestations. This may be particularly useful for differentiation from smoldering myeloma [10]. FDG-PET studies can also provide information on extramedullary foci and therapeutic response, but are not currently a standard investigation [11]. At the latest before initiating therapy, an MRI of specific regions should be ordered if extramedullary involvement is suspected or if neurological symptoms are present. Echocardiography is also indicated when cardiac amyloidosis is suspected [3].
Increasingly, genetic diagnosis is playing a significant role in the management of multiple myeloma. Analyses on del(17p), t(4; 14), and t(14; 16) correspond to the minimum work-up today [3]. These genetic alterations are associated with a significantly worse prognosis. Other aberrations and also gene expression-based prognostic scores are prognostically relevant but currently not (yet) predictive of specific therapies [6,12].
Classification for estimating the prognosis
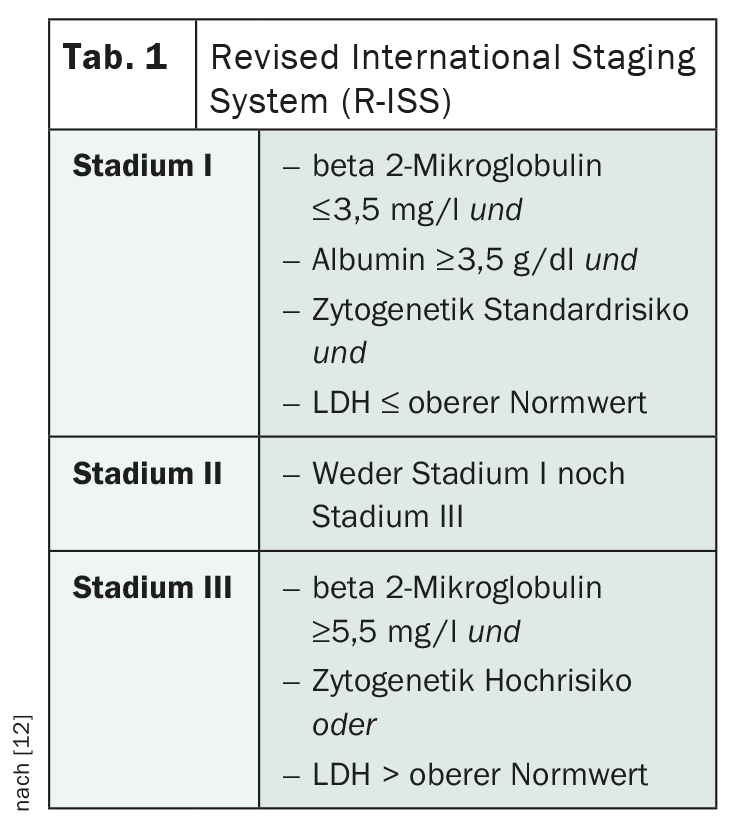
Staging of multiple myeloma plays a significant role in estimating prognosis and evaluating the most appropriate therapy. The classification according to Salmon and Durie, which has been in use for a long time, is now obsolete. In its place, the Revised International Staging System (R-ISS) of the International Myeloma Working Group ( IMWG) is used (Table 1) [12]. Based on this staging, affected individuals are divided into three prognostic groups, taking into account serum albumin, beta 2-microglobulin, LDH, and cytogenetic aberrations.
The presence of minimal residual disease (MRD) after therapy is also prognostically significant. It is present in the vast majority of patients even after achieving complete remission by current criteria and correlates with worse outcome [13]. Because MRD analyses have not yet been shown to have predictive value for further therapy, they are not currently a standard of care in follow-up studies [3].
First-line therapy at a glance
In addition to symptomatic courses and the so-called CRAB criteria (hypercalcemia – C, renal insufficiency – R, anemia – A, and bone involvement – B), there are also other radiological as well as serological parameters that represent indications for the initiation of therapy according to the IMWG criteria. These include evidence of myeloma-defining biomarkers, a clonal plasma cell content in the bone marrow of >60%, a serum free light chain quotient of >100 (affected/non-affected light chain), and focal lesions greater than 1 cm on MRI [3].
If there is an indication for therapy, a primary distinction is made between those patients who are suitable for stem cell transplantation and those who must be treated without high-dose therapy due to comorbidities. If transplantation is not an option, various two- and three-drug combinations can be used in drug treatment. Affected individuals with renal insufficiency, high disease activity, and unfavorable cytogenetics should preferentially receive therapy with bortezomib as a component [3]. Other agents used include cyclophosphamide, melphalan, lenalidomide, and steroids. Unfortunately, only a few of the common schemes were directly compared. Overall, combinations of three agents using a proteasome inhibitor, an immunomodulator, and a steroid appear to be superior to dual combinations. Therefore, if the general condition is good, these are preferable [3].
If autologous stem cell transplantation is available, it is the treatment of choice in the first line of therapy. To date, no new drug has been able to match transplantation in terms of remission rate, depth of response, and progression-free survival [14,15]. The limiting factor for the indication of autologous stem cell transplantation is the adverse effects of high-dose therapy. This requires good organ function and the absence of significant comorbidities [16]. Only a few years ago, vincristine-anthracycline-containing induction therapy was recommended. This has since been replaced by combination therapies with the newer agents thalidomide, bortezomib and lenalidomide, which lead to significantly better response rates. Again, data for direct comparison of the different combination therapies are unfortunately limited; complete remissions are achieved in 20-40% of cases [3]. Currently, the choice of induction therapy is primarily based on individual patient factors, side effects, and drug availability.
Stem cell transplantation following induction therapy can be performed as a single or tandem transplant. In the latter, a second autologous transplant is performed within six months. This leads to a prolongation of progression-free survival, and in patients with R-ISS stage III, a longer overall survival was observed in addition to a longer progression-free survival [17]. However, the increased toxicity of a second high-dose therapy must also be considered. Currently, with the inclusion of various subgroup and long-term analyses, tandem transplantation is recommended for patients from R-ISS group III and for those affected with high-risk cytogenetics [3]. Of course, the presence of a sufficient number of preserved autologous stem cells must be ensured. For high-dose therapy, melphalan 200 mg/m2 is used today [18]. Alternative conditioning regimens involving the use of cyclophosphamide and/or total body irradiation are no longer recommended due to their higher toxicity. Optimal high-dose therapy is also currently still the subject of various studies; additional administration of busulfan did not result in a prolongation of overall survival. Unfortunately, the addition of bortezomib also did not prove to be effective [19]. No conclusive data exist on the implementation of consolidation therapy after autologous stem cell transplantation; this may be particularly useful in patients with suboptimal response after transplantation or with high-risk cytogenetics [20].
The recommendations for maintenance therapy are clearer. After years of not being recommended, it is now an important part of the therapeutic standard – thanks to new drugs. Thus, bortezomib or lenalidomide is used in the high-risk group and lenalidomide is used for standard risk. And that’s even with complete response [3,19,21].
An important therapeutic factor that should not be forgotten regardless of transplant status is osteoprotection. Especially in cases of bone involvement and during glucocorticoid therapy, bisphosphonates or – particularly in cases of renal insufficiency – denosumab should be used to best prevent bone resorption. Zolendronate is considered the first-choice bisphosphonate in multiple myeloma [3].
What about progression or recurrence?
Progressions and relapses after first-line therapy are a major challenge in multiple myeloma. Not least because the patient population becomes even more inhomogeneous with later lines of therapy. Affected individuals have received different treatments and had different experiences. The choice of drugs is based, among other things, on first-line efficacy and tolerability. Thus, if the first-line experience is good, a drug of the same substance class can be used in the second-line treatment. However, if efficacy is low or tolerability poor, a change of drug class is indicated. Currently, there is a plethora of new drugs and drug combinations. Depending on the clinical presentation, previous therapies and comorbidities, a wide range of substances and sequences can thus be selected [3].
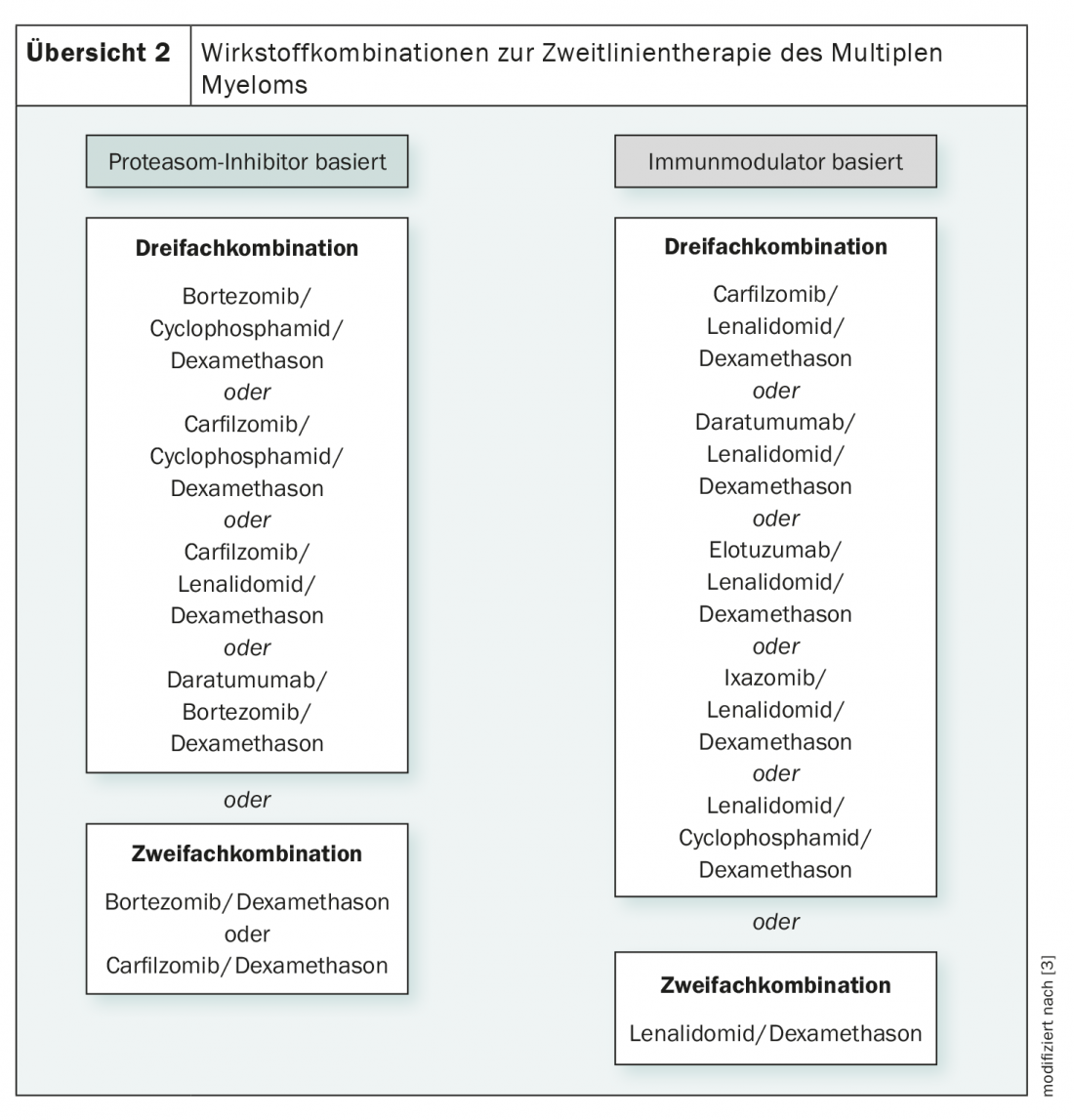
While early and late relapses are preferably treated by stem cell transplantation, diverse therapeutic regimens are available for all other relapses and for contraindications to transplantation. These are either proteasome inhibitor-based or immunomodulator-based and are composed of two to three active ingredients (overview 2) . The anti-CD38 antibody daratumumab is also used here. In general, triple combinations are more effective than double combinations, but more toxicities must also be considered [3,19].
With the increasing exploration of new agents for multiple myeloma therapy, some successes have been achieved in recent years, particularly in maintenance therapy and advanced lines of treatment. However, the prognosis in the high-risk group and in recurrent disease is still unfavorable today. Due to the increasingly widespread cytogenetic analysis, a better risk stratification is already possible. This raises hopes that in the future, not only diagnostic but potentially more targeted therapeutic options will be available.
Literature:
- Kyle RA, et al: Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003; 78(1): 21-33.
- NICER: National statistics on cancer incidence. www.nicer.org/en/statistics-atlas/cancer-incidence (last accessed Feb. 27, 2021).
- Wörmann B, et al: Multiple Myeloma. Onkopedia Guideline. www.onkopedia.com/de/onkopedia/guidelines/multiples-myelom/@@guideline/html/index.html (last accessed on 27.02.2021)
- Dimopoulos MA, et al: Pathogenesis and treatment of renal failure in multiple myeloma. Leukemia. 2008; 22(8): 1485-1493.
- Mikulasova A, et al: The spectrum of somatic mutations in monoclonal gammopathy of undetermined significance indicates a less complex genomic landscape than that in multiple myeloma. Haematologica. 2017; 102(9): 1617-1625.
- Bergsagel PL, Chesi MV: Molecular classification and risk stratification of myeloma. Hematol Oncol. 2013; 31 Suppl 1(0 1): 38-41.
- Landgren O, et al: Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009; 113(22): 5412-5417.
- Dispenzieri A, et al: Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010; 375(9727): 1721-1728.
- Rajkumar SV, et al: International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014; 15(12): e538-548.
- Dimopoulos MA, et al: Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol. 2015; 33(6): 657-664.
- Cavo M, et al: Role of (18)F-FDG PET/CT in the diagnosis and management of multiple myeloma and other plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017; 18(4): e206-e17.
- Palumbo A, et al: Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J Clin Oncol. 2015; 33(26): 2863-2869.
- Munshi NC, et al: Association of Minimal Residual Disease With Superior Survival Outcomes in Patients With Multiple Myeloma: A Meta-analysis. JAMA Oncol. 2017; 3(1): 28-35.
- Barlogie B, et al: Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997; 89(3): 789-793.
- Gay F, et al: Chemotherapy plus lenalidomide versus autologous transplantation, followed by lenalidomide plus prednisone versus lenalidomide maintenance, in patients with multiple myeloma: a randomised, multicentre, phase 3 trial. Lancet Oncol. 2015; 16(16): 1617-1629.
- Merz M, et al: Survival of elderly patients with multiple myeloma-Effect of upfront autologous stem cell transplantation. Eur J Cancer. 2016; 62: 1-8.
- Cavo M, et al: Double Autologous Stem Cell Transplantation Significantly Prolongs Progression-Free Survival and Overall Survival in Comparison with Single Autotransplantation in Newly Diagnosed Multiple Myeloma: An Analysis of Phase 3 EMN02/HO95 Study. Blood. 2017; 130 (supplement 1): 401.
- Giralt S: 200 mg/m(2) melphalan–the gold standard for multiple myeloma. Nat Rev Clin Oncol. 2010; 7: 490-491.
- Dimopoulos MA, et al: Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2021; 32(3): 309-322.
- Einsele H, et al: Response-adapted consolidation with bortezomib after ASCT improves progression-free survival in newly diagnosed multiple myeloma. Leukemia. 2017; 31(6): 1463-1466.
- McCarthy PL, et al: Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J Clin Oncol. 2017; 35(29): 3279-3289.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(2): 22-26.