Patients with moderate to severe atopic dermatitis suffer from the physical and psychological effects of the disease and yet do not receive adequate treatment with systemic therapies, or often only after a delay [1-3]. The Minimal Disease Activity approach aims to consistently identify patients who are candidates for advanced treatment, combining the treat-to-target concept with shared decision-making between patients and physicians [4].
Atopic dermatitis (AD) is characterized by eczematous skin lesions with severe itching and shows great heterogeneity in clinical features and severity [5]. In everyday life, the disease often represents a physical and psychological burden that restricts the quality of life (QOL) of those affected [1]. For patients with moderate to severe AD who are refractory to topical treatment, there are targeted systemic therapies that can reduce relapses and also alleviate psychosocial effects such as anxiety, depression and absence from work [6-8]. Nevertheless, only a minority (7-8%) of patients with moderate to severe AD receive systemic therapy [2, 3]. Delaying treatment can have a negative impact on the course of the disease, while early use of effective, systemic treatment options can prevent the lifelong effects of uncontrolled inflammatory skin disease [9-12].
Consensus for a treat-to-target approach for AD
With the collaboration of Swiss dermatologists Prof. Dr. med. Peter Schmid-Grendelmeier from the University Hospital Zurich and Prof. Dr. Peter Häusermann from the Dermatologie am Rhein Basel practice in a global program, the concept of the Minimal Disease Activity (MDA) with 100% approval for AD and now for the first time on World Congress of Dermatology 2023 will be presented at a symposium in Singapore. MDA is a new treat-to-target approach to identify patients with moderate to severe AD who are candidates for systemic treatment [4]. The MDA is defined as a level of disease activity that is considered a reasonable treatment goal by both patients and the treating physicians, taking into account the available treatment options [13]. This can optimize long-term treatment outcomes and be helpful for physicians when choosing treatment options [14, 15].

MDA concept in practice
In practice, the MDA criteria involve a combination of at least oneclinician-reported and onepatient-reported criterion. reported (patient-reported) outcome The selected targets are then listed according to priority (Fig. 1). In both categories, there are moderate goals that should be achieved within 3 months and, if not achieved, lead to modification or escalation of the therapy. Optimal goals should be achieved in the long term (from 6 months) and are considered MDA. The MDA approach combines the treat-to-target concept with shared decision-making between physicians and patients [4].
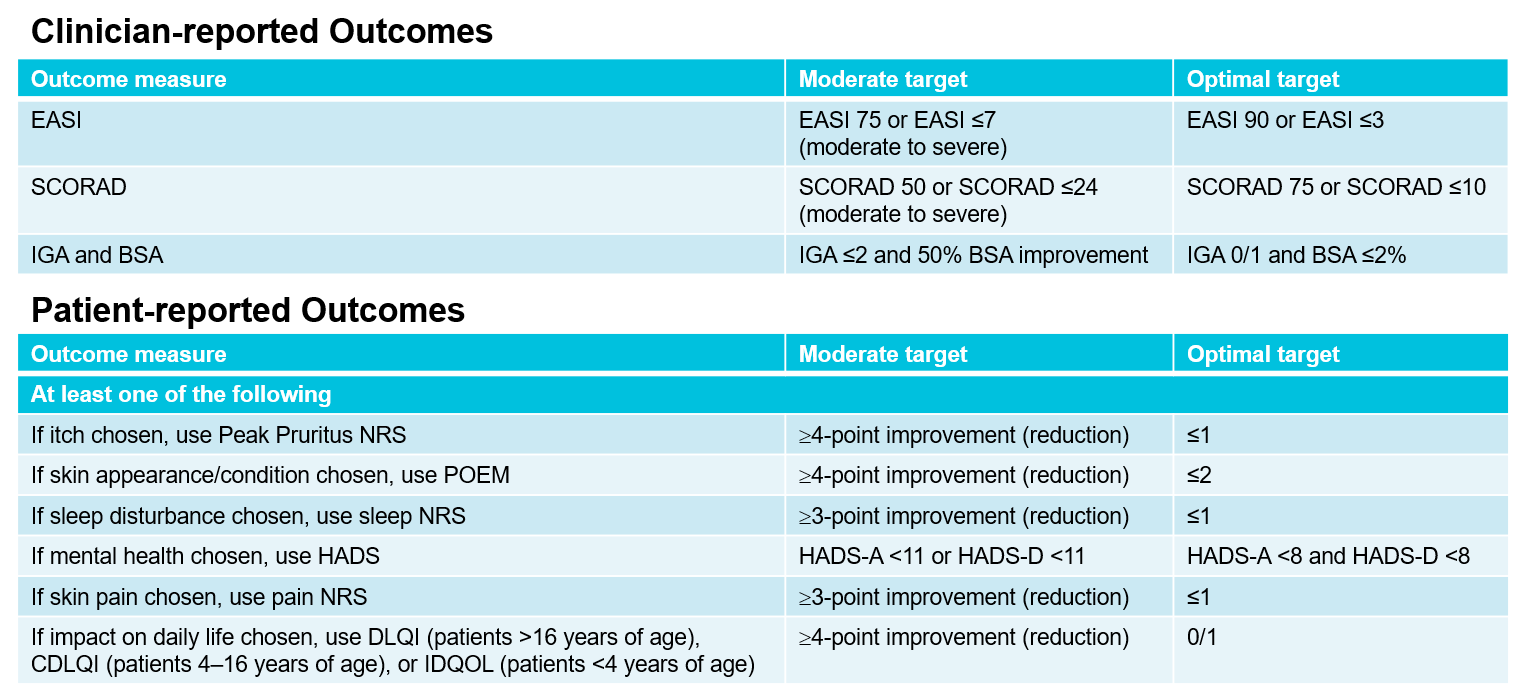
Fig. 1: Criteria for the MDA approach, which combinesclinically-reported and patient-reported outcomes. EASI, Eczema Area and Severity Index; SCORAD, SCORing Atopic Dermatitis; IGA, Investigator Global Assessment; BSA, Body Surface Area; NRS, Numerical Rating Scale; POEM, Patient Oriented Eczema Measure; HADS, Hospital Anxiety and Depression Scale; (C)DQOL, (Children’s) Dermatitis Quality of Life Index. Adapted from [4].
Achieving therapy goals
For patients, the most important therapeutic goals are relief of itching, improvement of symptoms and complete skin healing. The achievement of these patient-reported outcomes is also directly related to an improvement in QOL [1]. These treatment goals are now achievable thanks to the advanced treatment options for AD. For example, the oral JAK* inhibitor upadacitinib (RINVOQ®), which selectively inhibits JAK-1, achieved an EASI* 75 response in 82.0% of patients and an EASI 90 response in 62.7% of patients after 52 weeks in the pivotal, randomized, double-blind, placebo-controlled Phase III studies MEASURE Up-1 and MEASURE Up-2 with a dosage of 15 mg once daily [16]. In addition, a rapid response to RINVOQ® was observed – after just two days, significantly more patients reported significant relief from itching compared to patients on placebo [17]. RINVOQ® is indicated in the oral dosage of 15 mg once daily since November 2021 for the treatment of adults with moderate to severe AD when therapy with conventional topical medications does not provide adequate disease control or could not be used [18].
Conclusion
Suitable systemic therapies are available for moderate to severe AD to achieve high treatment goals [18-23]. MDA can be used to ensure that patients who are eligible for systemic treatment also receive it. It combines the treat-to-target concept and joint decision-making.
* JAK, Janus kinase; EASI, Eczema Area and Severity Index
Brief technical information RINVOQ®
Literature
1 Augustin, M., et al, Characterizing treatment-related patient needs in atopic eczema: insights for personalized goal orientation. J Eur Acad Dermatol Venereol, 2020. 34(1): p. 142-152.
2 Pascal, C., et al, Therapeutic management of adults with atopic dermatitis: comparison with psoriasis and chronic urticaria. J Eur Acad Dermatol Venereol, 2020. 34(10): p. 2339-2345.
3 Egeberg, A. and J.P. Thyssen, Factors associated with patient-reported importance of skin clearance among adults with psoriasis and atopic dermatitis. J Am Acad Dermatol, 2019. 81(4): p. 943-949.
4 Silverberg J, et al. Revolutionizing Atopic Dermatitis (RAD), Virtual Conference, December 11, 2022. Poster.
5 Langan, S.M., A.D. Irvine, and S. Weidinger, Atopic dermatitis. Lancet, 2020. 396(10247): p. 345-360.
6 Song, A., S.E. Lee, and J.H. Kim, Immunopathology and Immunotherapy of Inflammatory Skin Diseases. Immune Netw, 2022. 22(1): p. e7.
7 Silvestre JF et al. Real-World Burden in Patients With Atopic Dermatitis Who Are Candidates for Systemic Therapy and Currently Receiving No Systemic Therapy, No Treatment, Topical Therapy Alone, or Systemic Therapy: Results From a Real-World Multicountry Study. EADV, Milan, September 7-11, 2022. Oral session FC02.02.
8 Megna, M., et al, Systemic Treatment of Adult Atopic Dermatitis: A Review. Dermatol Ther (Heidelb), 2017. 7(1): p. 1-23.
9 Marzano, A.V., et al, Evidence for a ‘window of opportunity’ in hidradenitis suppurativa treated with adalimumab: a retrospective, real-life multicentre cohort study. Br J Dermatol, 2021. 184(1): p. 133-140.
10 Kimball, A.B., et al, Psoriasis: is the impairment to a patient’s life cumulative? J Eur Acad Dermatol Venereol, 2010. 24(9): p. 989-1004.
11 Linder, M.D., et al, Psoriasis – The Life Course Approach. Acta Derm Venereol, 2016. 96(217): p. 102-8.
12 Ros, S., L. Puig, and J.M. Carrascosa, Cumulative life course impairment: the imprint of psoriasis on the patient’s life. Actas Dermosifiliogr, 2014. 105(2): p. 128-34.
13 Wells, G.A., et al, Minimal disease activity for rheumatoid arthritis: a preliminary definition. J Rheumatol, 2005.32(10): p. 2016-24.
14 De Bruin-Weller, M., et al, Treat-to-Target in Atopic Dermatitis: An International Consensus on a Set of Core Decision Points for Systemic Therapies. Acta Derm Venereol, 2021. 101(2): p. adv00402.
15 Vestergaard, C., A. Wollenberg, and J.P. Thyssen, European Task Force on Atopic Dermatitis (ETFAD) Position Paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol, 2020. 34(2): p. 426-427.
16 Simpson, E.L., et al, Efficacy and Safety of Upadacitinib in Patients With Moderate to Severe Atopic Dermatitis: Analysis of Follow-up Data From the Measure Up 1 and Measure Up 2 Randomized Clinical Trials. JAMA Dermatology, 2022. 158(4): p. 404-413.
17 Guttman-Yassky, E., et al, Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomized controlled phase 3 trials. Lancet, 2021. 397(10290): p. 2151-2168.
18. current RINVOQ® (upadacitinib) prescribing information at www.swissmedicinfo.ch
19. current technical information Sandimmun Neoral® (cyclosporin A) at www.swissmedicinfo.ch.
20. current technical information DUPIXENT® (dupilumab) at www.swissmedicinfo.ch.
21 Current OLUMIANT® (baricitinib) prescribing information at www.swissmedicinfo.ch.
22. current ADTRALZA® (tralokinumab) prescribing information at www.swissmedicinfo.ch.
23 Current information for healthcare professionals CIBINQO® (abrocitinib) at www.swissmedicinfo.ch.
The references can be requested by professionals at medinfo.ch@abbvie.com.
This article was produced with the financial support of AbbVie AG, Alte Steinhauserstrasse 14, Cham.
CH-RNQD-230040_11/2023
This article has been released in German.
Contribution online since 15.09.2023
Post updated: 11/17/2023












