Over time, an almost unmanageable number of new wound treatments and wound dressings have accumulated. Within this article, the authors present the principles of local wound treatment as well as some newer treatment methods.
In order to be able to treat the underlying pathology adequately, a clarification of the cause is the conditio sine qua non of any ulcer therapy before starting any wound treatment. Numerous studies show that treatment of the causative factors is much more important than local therapy to allow healing.
The goal of any local wound treatment is to create an optimal wound environment in which a well-perfused, stable wound bed with little exudate is created. This is achieved by removing senescent or abnormal cells, reducing the bacterial load, reducing wound exudate, and promoting the formation of granulating and epithelializing tissue. In English, these principles of wound care are known by the acronym T.I.M.E. (tissue management, inflammation and infection control, moisture balance and epithelial (edge) advancement).
Debridement
A chronic wound stagnates in the inflammatory phase of the wound healing cascade. Thus, a local milieu exists in the wound, which allows healing only to a limited extent. Fibrin coatings and necrosis inhibit wound healing and provide a breeding ground for microorganisms.
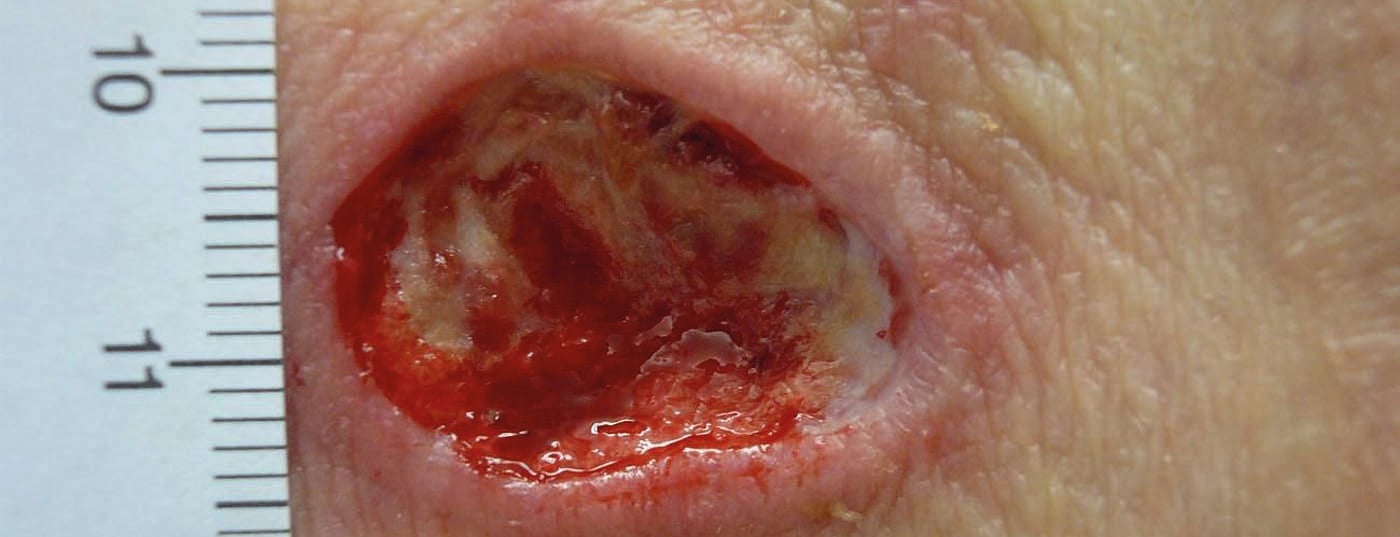
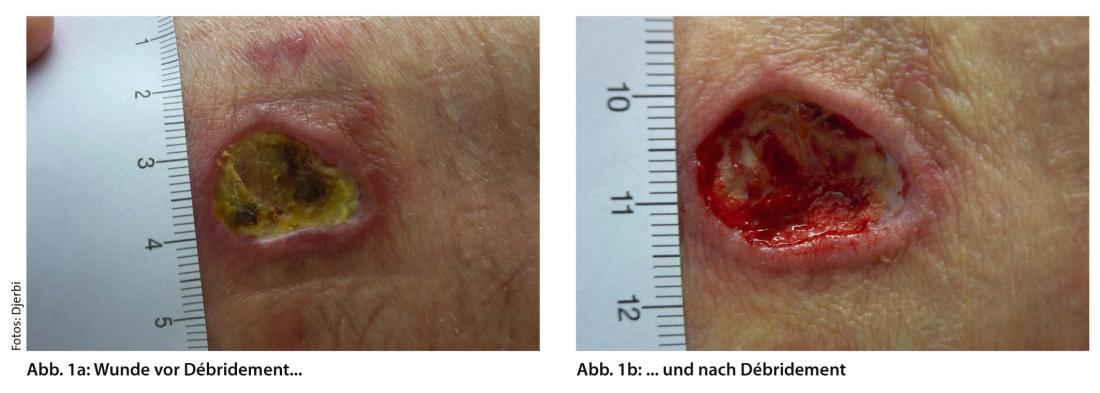
Debridement removes avital, infected and damaged tissue to promote wound healing and minimize the risk of infection (Fig. 1a and b).
In principle, debridement can be performed on all wounds, with the exception of pyoderma gangraenosum, in which wound enlargement could be induced due to the pathergy phenomenon. A distinction is made between different forms of debridement (Tab. 1). The debridement method depends on the patient’s condition, the wound status, the skills of the wound team and the available resources.

Inflammation and infection control
The microbial load of a wound can range from colonization to local infection (critical colonization) to infection with tissue infiltration or systemic reaction. More and more work is showing that biofilms form on chronic wounds and that these impair wound healing. It is recommended to take a microbiological swab of each new wound in order to be able to start an adequate treatment according to the antibiogram and to exclude multiresistant germs in case of a corresponding suspicion of colonization or infection.
For wound treatment, antiseptics and antibiotics can reduce the bacterial load. In this context, antiseptics have a broader antimicrobial spectrum and resistance, which is a major problem with selectively active antibiotics, is rare. In addition, locally applied antibiotics carry the risk of allergic sensitization.
Some antiseptics such as concentrated PVP iodine are tissue toxic and inhibit granulation tissue formation. When using the most common antiseptics, such as octenisept and polihexanide, pay attention to the exposure time. While Octenisept achieves the disinfecting effect after only one minute, Polihexanid (PHMB) must act for at least 10-15 minutes.
There are multiple wound dressings that contain integrated antiseptic substances such as silver, PHMB, iodine or chlorhexidine.
In addition to classic signs of infection, patients with chronic wounds should be watched for discrete signs of local infection such as increasing pain, wound size progression, increased exudate, excessive easily vulnerable reddish granulation tissue, or stagnation in the healing process. If there are clear clinical signs of infection, such as systemic symptoms, redness, or hyperthermia, systemic antibiotics must be used.
Moisture balance and epithelization promotion
It has been known for several decades that wounds heal more rapidly in a moist environment; this favors the activity of growth factors, cytokines and cell migration. In addition, the moist wound environment favors autolytic debridement and thus facilitates wound debridement. However, if there is too much wound exudate, this can destroy newly formed tissue again due to a predominance of proteases and inhibit wound healing processes as well as macerate the surrounding skin.
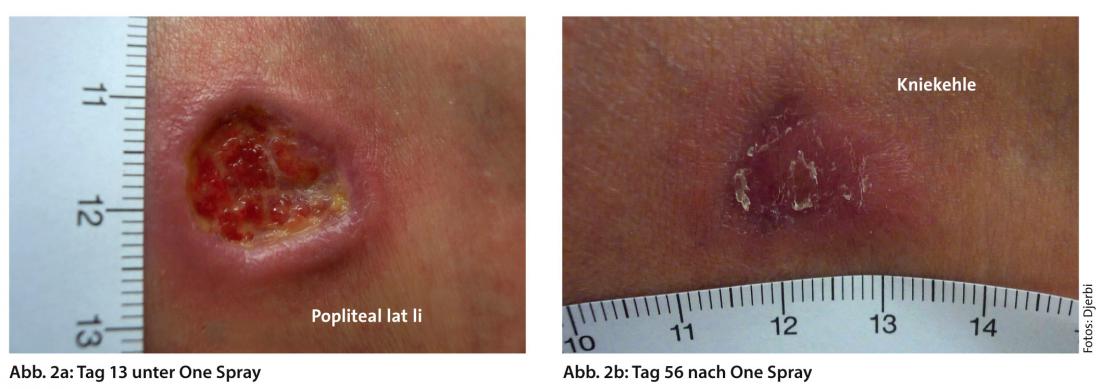
Wound dressings should therefore provide an adequate moisture balance and prevent both maceration and drying of the wound bed (Fig. 2a and b).
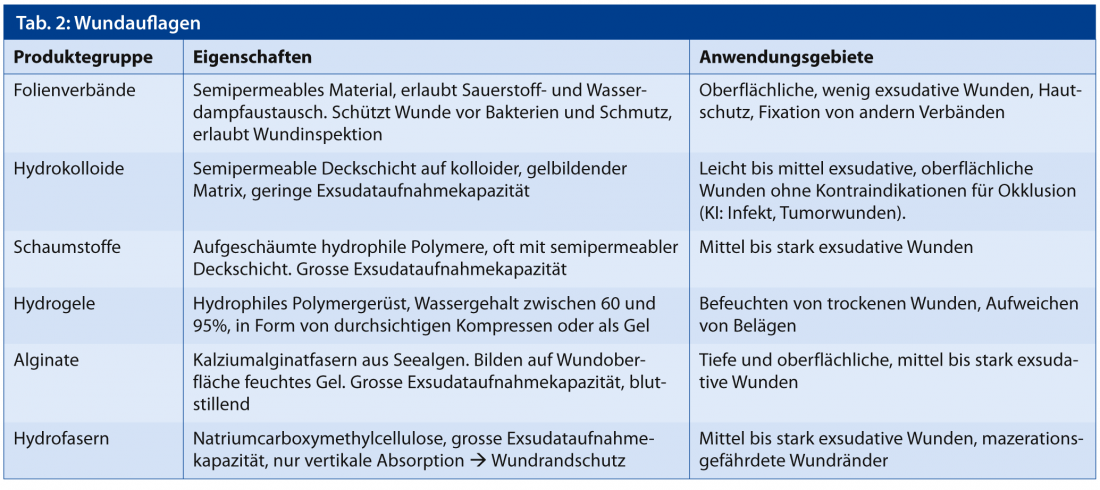
The choice of dressing is critical in this regard, as some dressings can absorb large amounts of moisture (alginates, foams), while others tend to retain or release moisture (hydrocolloids, hydrogels). Dressings can reduce quality of life limiting factors such as pain, excessive exudation and fetor. Table 2 lists a summary of the most commonly used wound dressings.
In addition to the commonly used wound dressings, there are other newer technologies and materials on the market that are intended to be used primarily for delayed wound healing. These include negative pressure therapy, collagen matrices, vegetable oils and live skin equivalents.
A proven technology, especially for highly exudative wounds, is negative pressure therapy (also called “vacuum assisted closure therapy” or “negative pressure wound therapy”). After applying a foam to the wound, the wound secretion is regularly aspirated by means of a pump. This method leads to the reduction of excessive exudation, edema, microorganisms and promotes the formation of granulation tissue and blood circulation. However, this wound treatment requires a certain infrastructure and experience. While clinical experience shows a sometimes impressive induction of granulation tissue, the evidence base for faster wound closure is not clear for all wound types.
Newer therapy options
As mentioned above, it is believed that the excessive formation of proteases inhibits wound healing. For this reason, wound dressings such as the protease-modulating matrix (Promogran®, Promogran Prisma®, Urgo Start®) have been developed to reduce this activity. In order to make a better therapy decision, it is helpful to perform a protease rapid test.
A three-dimensional collagen matrix of porcine small intestinal mucosa, which facilitates cell ingrowth, can also lead to accelerated healing in various types of ulcers. Both matrices are completely absorbed and do not need to be removed during dressing changes.
Another new therapeutic option is the application of a mixture of neem oil and St. John’s wort oil. Initial clinical experience shows impressive induction of granulation tissue for these herbal substances, especially in postoperative wounds. However, there are no proven studies for this yet.
A well-established treatment method is autologous skin grafting of split thickness or full thickness skin. However, as this involves a great deal of effort and cannot be performed on every patient, new skin equivalents such as Epidex® and Apligraf® have been developed.
While Epidex® is cultivated from the patient’s own hair follicle cells, Apligraf® is derived from non-patient newborn foreskin. Some studies showed a benefit of these living skin equivalents for faster wound healing compared to conventional wound dressings.
However, due to the high cost, SAfW and SGDV guidelines must be considered before using skin equivalents and collagen matrix to ensure reimbursement by health insurance (www.safw.ch/index.php/aktuell/bag-richtlinien).
Conclusion for practice
Before any wound treatment, it is necessary to clarify the cause. In principle, avital tissue should be removed (debridement), infection and inflammation controlled, and moisture balance established. To maintain a moisture balance, the rule is roughly wet on dry and dry on wet. In addition to causal therapy, important adjunctive treatments include adequate analgesia, pressure relief, and appropriate/adequate nutrition.
We recommend that every practitioner become familiar with a selection of wound dressings. For the newer wound dressings, the Swiss guidelines should be considered (www.safw.ch).
Nadia Djerbi, MD
DERMATOLOGIE PRAXIS 2013; 23(3): 6-8