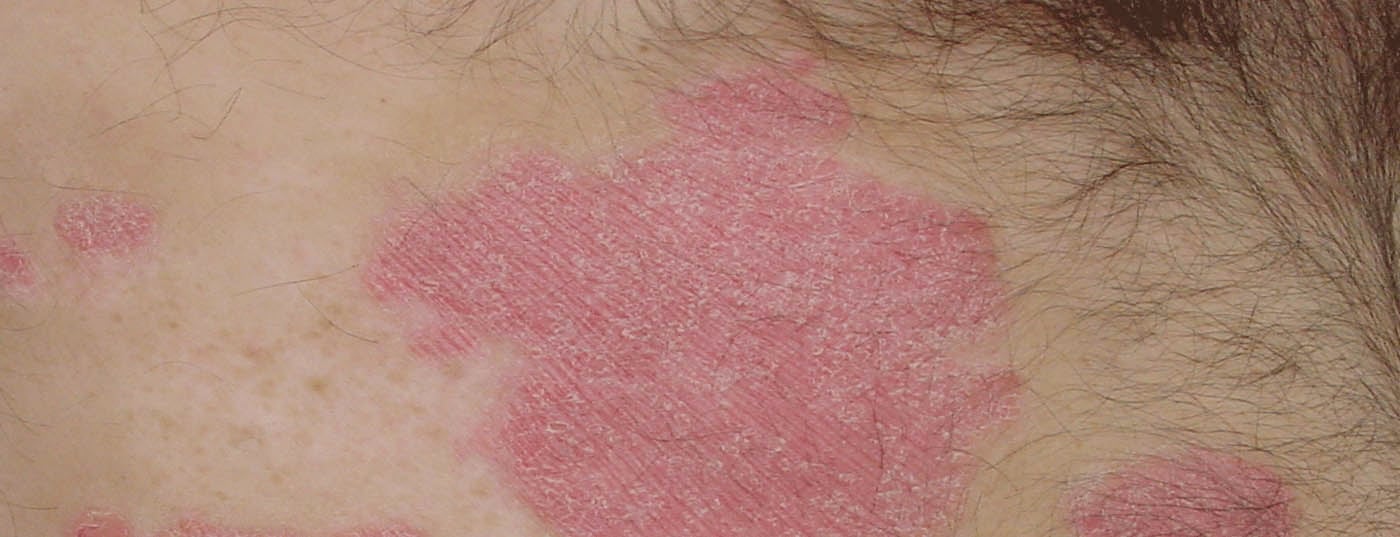
In an interview with DERMATOLOGIE PRAXIS, Dr. med. Simon Müller, senior physician in dermatology at the University Hospital Basel, talks about the topic “Therapy of plaque psoriasis”. In doing so, he provides a comprehensive overview of developments in recent years, discusses the advantages and disadvantages of the biologics era, and talks about his experiences in the clinic. Patient selection, the “psoriatic march” and adherence are also addressed.
Dr. Müller, how do you personally assess the developments in psoriasis therapy since the turn of the millennium? Or more specifically, what decisive advantages and disadvantages did the era of biologics bring for you as a clinician?
Dr. Müller:
The introduction of biologics spurred both basic and clinical research on psoriasis. As a result, great progress has been made in understanding the pathogenesis, but also in treatability. The enormous research activity in psoriasis over the last 15 years is certainly due in large part to the huge global market volume, due in part to the prevalence of about 2% in the normal population and chronicity.
Meanwhile, as a clinician, I almost have a luxury problem: I can choose between several well and very well effective system therapies. It can be difficult to keep track and decide which therapy is most suitable for which patient. However, in addition to their good efficacy and lower organ toxicity compared to methotrexate or ciclosporin, biologics also brought us a new side effect profile and a multiplication of drug costs compared to these conventional systemic therapies – both aspects I have to consider as a clinician.
How do you decide at the dermatology clinic in Basel when to use local, UV or a system therapy (do you strictly follow the severity or the PASI/BSA/DLQI values)?
In principle, we already follow the recommendations of the European Consensus Program of 2010 – i.e. PASI >10 or BSA >10, provided that the DLQI is also >10. Ultimately, of course, the patient’s needs and individual factors such as comorbidities are also taken into account in treatment planning. The recommendations also include “soft” criteria for special situations that allow a certain degree of leeway, such as resistance to treatment of small areas, nail, genital or palmoplantar infestation. The evaluation of a system therapy in patients with psoriatic arthritis, children, pregnant women, patients with a consumptive or infectious disease, dementia or malcompliance takes place in our team or in our practice. interdisciplinary takes place.
How does the Dermatology Life Quality Index (DLQI) perform as a “decision-making tool” in severity classification? In your opinion, is it reasonable to value subjective disease assessment so much over clinical physician assessment with regard to treatment decisions?
In most studies, PASI-75 response or, in more recent studies, PASI-90 response or even clearance is measured as the primary endpoint. This is also useful so that studies can be compared at least approximately objectively. On the other hand, the PASI does not reflect the patient’s subjective assessment, resulting in a biased physician-centered severity assessment. That’s why I personally consider the DLQI as a “decision-making tool” to be very useful, because a PASI-50 response may already bring more subjective improvement for one patient than, for example, a PASI-75 response for another. Combine this with the fact that although the PASI is suitable for clinical trials, in my experience it is not always practical in everyday clinical practice and is subject to significant interrater variability.
The fact that the DLQI is rated even higher than the PASI according to the above-mentioned European Consensus Program is, however, controversial because under-treated patients have an increased risk of an accelerated psoriatic march. an increase in psoriatic arthritis would be possible. In addition, the DLQI only asks about the last seven days, so it may only be an inadequate snapshot.
Regarding local therapy, how and with what advantages can topical corticosteroids be combined with vitamin D3 analogues or with salicylic acid?
Combination therapy of topical corticosteroids with vitamin D3 analogs or salicylic acid is more potent than the respective monotherapy and is steroid-sparing. For vitamin D3 analogs, it is important to note that a maximum of one 100-g tube per week should be used because of the potential for hypercalcemia. According to the drug compendium, they should not be used in children due to lack of experience, although no age limit is defined. The keratolytic effect of salicylic acid is useful as long as significant scaling is present, in part to improve the penetration of topical corticosteroids. In children, large-scale application of salicylic acid should not be performed because of potential neurotoxicity and nephrotoxicity.
What is the importance of UV therapy today (especially since the introduction of biologics)?
UV therapies have been a mainstay of therapy for moderate to severe psoriasis for decades. However, as we have been able to show on the basis of our own data, their importance has decreased significantly since the introduction of biologics for this indication. In our company, treatments with UV therapy have roughly halved in the last 15 years, and we have not performed any system PUVA at all for several years. However, UV therapy still has an important role in the mild forms or initially in moderate to severe forms until the onset of action of systemic therapy. Further combination with systemic therapy is contraindicated because of the potentially increased risk of photocarcinogenesis. Occasionally, UV therapy can still offer an alternative to systemic therapy – for example, during pregnancy or in certain contraindications such as severe hepatopathy.
What new developments or active ingredients are there in the field of biologics and small molecules, and how do you classify them?
In the last three years, several of these products, such as the IL-17 antagonists secukinumab and brodalumab, or the small molecule apremilast, a phosphodiesterase-4 inhibitor, and tofacitinib, a Janus kinase inhibitor, have reached market maturity and, in some cases, have already received approval for psoriasis and psoriatic arthritis in some countries. Of these, only Cosentyx® (secukinumab) is currently approved in Switzerland. The others will probably also receive approval in this country in the next year or two.
The marketing authorization of brodalumab took an unexpected turn on May 22, 2015. Due to reports of suicidal thoughts and actions during the “open-label extension” phase, Amgen has suspended further development of brodalumab effective immediately. Responsibility for brodalumab now rests with AstraZeneca and the future of this product is uncertain. After Efalizumab and Briakinumab, this is another example of how a biologic can be abruptly withdrawn due to safety concerns shortly before or even after market approval.
Numerous other biologics and small molecules are currently undergoing Phase II and III studies and are likely to keep us busy in the medium term. The true value of all these new products will only become clear after five to ten years of market experience. In clinical trials, however, at least some of them show promise, possibly acting more rapidly and efficiently than established biologics. Oral therapies such as apremilast may also offer new benefits.
In addition to the new system therapies, Janus kinase inhibitors, phosphodiesterase inhibitors and new vitamin D analogues are currently being investigated as topical agents in clinical trials. There are some voices saying that market saturation has already been reached in antipsoriatics – and there are still some new ones in the pipeline. Thus, it is becoming increasingly difficult for a single product to stand out from the rest. Perhaps this market saturation will have a favorable effect on the prices of these therapies from a health economic perspective, which would be welcome. New perspectives, especially with regard to pricing, are also to be expected with the so-called biosimilars, which will stimulate the market for antipsoriatics in the next few years when patent protection for etanercept and adalimumab expires.
How frequently is methotrexate used in your clinic (compared to the other conventional systemic therapeutics or the biologics)?
Methotrexate is well-tried, very often effective with good tolerance and at the same time the most cost-effective of all system therapeutics in psoriasis. Therefore, it is still our first-line therapy of choice for moderate to severe psoriasis – contraindications included. Pregnancy excluded.
How do you decide which biologic is most appropriate for which patient?
Essentially using the following six questions: 1. is there psoriatic arthritis?; 2. is the patient obese?; 3. is a rapid start of therapy most important?; 4. is maximum efficacy (e.g. due to erythroderma) most important?.; 5. how good is the compliance?; 6. are breaks in therapy foreseeable?
The “Swiss recommendations for biologics treatment in psoriasis” then help to find the appropriate biologic depending on the situation. These were established in 2011, and in the meantime ustekinumab has also received approval for psoriatic arthritis in Switzerland, and the European Medicines Agency has positively assessed the use of adalimumab in children with psoriasis aged four years and older. Where the newly launched secukinumab will position itself under everyday conditions remains to be seen, but the superiority shown over etanercept and ustekinumab in phase III studies is at least promising.
How effective are the individual biologics approved for psoriasis in short- and long-term comparisons (are there even enough head-to-head studies in this area)?
Interpreting comparisons of the effectiveness of biologics is complex, and each manufacturer can cite at least one study that says why its own product is the best. One problem is that there are not enough head-to-head studies. This will change, however, as the new biologics and small molecules must confirm their raison d’être in such head-to-head comparisons. For established biologics, PASI-75 response is often compared from different studies with different study designs. In this context, most randomized-controlled pivotal trials are limited to a period of 12-24 weeks. After that, comparability becomes even more difficult because of comparisons of combination therapies, dose differences, uncontrolled registry data, and different endpoints.
As a rule of thumb, I assume that in the first four to six months infliximab is the most effective biologic, etanercept (2× 50 mg/week) rather the weakest, and adalimumab and ustekinumab somewhere in between. However, treatment adherence and loss of efficacy, which occurs in approximately one-quarter of patients, are also critical to long-term efficacy. Etanercept tends to make up ground here.
Your personal experience: Are biologics safe to use and are they well tolerated? Is it necessary to distinguish between the TNF-alpha inhibitors and the interleukin antibodies in this regard?
I experience the tolerance and safety of both TNF alpha inhibitors and ustekinumab as very good overall, and see some weaknesses in some cases rather in the efficacy. Personally, I have not witnessed any of the much discussed potentially serious side effects of biologics such as severe infections, autoimmune phenomena, “major cardiovascular events”, demyelinating diseases or malignancies in our patients. On the one hand, this has to do with the fact that we carefully select the patients. In addition, we are exhausting conventional system therapeutics and therefore perhaps treat fewer patients with biologics than other university hospitals. The main reason, however, is that these serious side effects are very rare, with five to eight events per 100 patient-years, and only become measurable in larger populations.
How does the application of local substances actually work? In some cases, up to three different active ingredients have to be applied daily (corticosteroids + vitamin D3 analogues or salicylic acid + lipid-replenishing products). Is the patient only concerned with skin care or does adherence not suffer greatly with such combination therapies?
The practicality and acceptability of local therapy is of critical importance, otherwise it will not be used. I recommend re-fatting in the morning after showering, and medicinal local therapy in the evening at least 30 minutes before sleeping. For the first two weeks, I recommend a combination product such as Daivobet® ointment/gel for this purpose, alternating daily with e.g. Diprosalic® ointment in the case of thick scaling. Using topical corticosteroids twice is not recommended, as this only increases the side effects. If necessary, I recommend occlusion with cellophane film. After these two weeks, topical corticosteroids can be reduced to about 2-3×/dweek and instead vitamin D3 analogues can be used as monotherapy on these days along with refatting. For intertriginous areas, topical calcineurin inhibitors 1-2×/d are recommended.
What is the so-called “psoriatic march” and what consequences does it have for therapy?
This refers to the concept that psoriasis, as an inflammatory disease, also “goes under the skin” and does not only induce inflammatory processes at the joints and tendon attachments. Because of proinflammatory cytokines, it also leads to insulin resistance, which can cause endothelial dysfunction with atherosclerosis and ultimately cardiovascular events. There is increasing evidence that psoriasis may be an independent cardiovascular risk factor. However, the proof of concept is still pending and is complicated by the fact that many psoriasis patients also have the other classic cardiovascular risk factors. Obesity per se may also lead to upregulated proinflammatory cytokines.
So the “psoriatic march” is still somewhat hypothetical, but to me it’s a pointer to the good control of cardiovascular risk factors in psoriatic patients because they had increased cardiovascular morbidity and mortality in some large epidemiological studies. However, there were also studies with contrary results, so the data situation is still controversial.
Interview: Andreas Grossmann
DERMATOLOGIE PRAXIS 2015; 25(3): 10-13