While targeted therapies are on the rise, there are still tumors that cannot (yet) benefit from this development. One such is triple-negative breast carcinoma. But even here, far from targeted therapies, new insights are constantly being gained. For example, immunotherapy with checkpoint inhibitors appears to be a promising option.
Already contained in the term “triple-negative breast cancer” are the two main difficulties for the therapy of this entity: It is a catch-all of tumors defined by what does not constitute them, and known targets for targeted treatments are lacking. As triple-negative breast cancer still accounts for 15-20% of all newly diagnosed breast cancers, well-established and effective treatment regimens are of great importance despite these challenges. 95% of diagnoses are made in stages I-III, and only 5% of triple-negative breast carcinomas are already metastatic at diagnosis. Currently, 20% relapse during the course of the disease – a number that must be further reduced by optimizing management. Because even though much has been done, the outlook for triple-negative breast cancer is still the worst among breast cancers.
Analysis of a colorful pile
Triple-negative breast carcinomas are a heterogeneous group of tumors whose common feature is that they do not express estrogen or progesterone receptors, nor HER2. In general, the proliferation rate is usually higher than in other breast cancer types with a correspondingly poorer prognosis. Most triple-negative breast carcinomas are ductal, but other histologic types exist. Since these differ in terms of optimal treatment, careful histological diagnosis is of high importance. For example, adenoid cystic carcinoma has a good prognosis and does not necessarily require chemotherapy.
So we are dealing with different opponents, which also differ molecularly. To this end, Lisa Carey from the University of North Carolina presented the CALGB 40603 study at this year’s San Antonio Breast Cancer Symposium, which, among other things, looked at the molecular characteristics of different forms of triple-negative breast cancer. She stressed the importance of such analyses, which not only serve to stratify risk but could also provide therapeutic targets in the future. Depending on the typing method, different proportions of luminal, mesenchymal and basal tumor subtypes were revealed.
A question of the right timing
In the absence of molecular targets, chemotherapy remains the focus of treatment in triple-negative breast carcinoma. Here, the efficiency of the therapy is independent of the time of administration. Thus, the drug effect remains the same whether the chemotherapy is adjuvant or neoadjuvant. A decisive advantage of neoadjuvant therapy: downstaging is possible. In one study, this allowed 42% of patients who did not originally qualify for breast-conserving surgery to undergo breast-conserving surgery after all. And with a success rate of 91% [1].
The potential downstaging from neoadjuvant chemotherapy also affects axillary lymph node involvement. This is extremely relevant clinically, Lisa Carey explained in her presentation, as 10 to 20% of all patients developed lymphedema after axillary dissection. By avoiding this operation, the often serious complication could also be prevented. Sentinel lymph node biopsy after neoadjuvant treatment would need to be performed somewhat more carefully using a dual tracer and with sampling of at least three lymph nodes, but would be reliable, he said. Currently, two clinical trials are investigating axillary conversion in some detail. On the one hand, we address the question of whether radiation could replace axillary dissection in the future for patients who still have axillary involvement after neoadjuvant chemotherapy. On the other hand, researchers are currently investigating whether radiotherapy outside the breast is even necessary in the event of lymph node status conversion.
Not only the timing of chemotherapy is crucial for the further course, but also its frequency. So it depends on the intervals at which the medication is administered. In the past, significantly better results could be achieved by increasing the so-called “dose density” [2]. Thanks to the development of growth factors, it was possible to reduce the duration between the individual drug administrations and thus, among other things, to sustainably reduce the risk of recurrence.
Stages I-III: Choice of drugs
Over the past years and decades, the therapeutic standard for adjuvant and neoadjuvant treatment of triple-negative breast carcinoma has improved significantly. Carey demonstrated this by comparing recurrence rates in the periods from 1986 to 1992 and from 2004 to 2008. In the second time interval, early recurrences within the first six years after diagnosis in particular were significantly less frequent. These decreased by 25% to 45%, but still account for the largest proportion of recurrences.
Even older therapy regimens were based on the use of anthracyclines, which are still the basis of chemotherapy today. Over time, taxanes in particular were added. The benefit of adding paclitaxel was demonstrated in the CALGB 9344 trial in 2007 and profoundly changed treatment and prognosis. Thus, the expansion of therapy increased disease-free survival (DFS) by 15% to 20% in absolute terms. Likewise, the addition of blanks was investigated several times. While several independent studies confirmed a benefit of additive medication on response rates [3,4], the effect on recurrence risk cannot yet be conclusively assessed, according to Carey. This was due to different basic therapies in the studies, some of which did not correspond to the current standard. Until further results are available, the expert recommends the use of carboplatin in patients with positive lymph node status and patients who have unfavorable prerequisites for breast-conserving surgery. This could reduce the rate of axillary dissection and increase the number of patients eligible for breast-conserving therapy.
Response to neoadjuvant drug therapy, unsurprisingly, plays a significant role in outcome. This is also supported by 5-year follow-up data from the CALGB-40603 trial, which additionally shows that the extent of response also has prognostic significance. A recent study demonstrated that in the case of an incomplete response to neoadjuvant chemotherapy, the additional administration of capecitabine for six months provides significant and long-lasting benefits in terms of overall survival and recurrence rate [5].
Efforts to remove anthracyclines from therapy because of their risk of cardiac failure and leukemia have been less successful. All alternatives tested to date have been shown to be inferior, particularly in the treatment of nodal positive tumors [6].
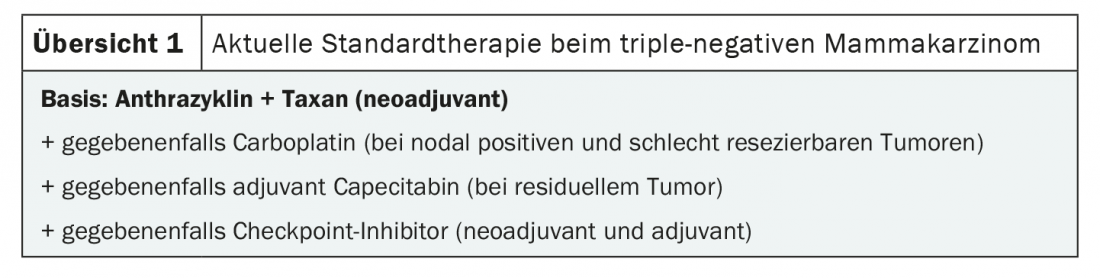
The bottom line is that anthracyclines remain the basis for chemotherapy in non-metastatic triple-negative breast cancer (overview 1) . In addition, taxanes and, in the neoadjuvant setting, carboplatin may be added for more advanced tumors. If residual tumor remains after surgery, administration of capecitabine is indicated. By default, chemotherapy is started neoadjuvantly, with an exception for very small tumors without lymph node involvement.
New wave: Immunotherapy
In line with current developments in oncology, various studies on the addition of immunotherapy to chemotherapy are ongoing. For example, while the KEYNOTE 522 and IMpassion 031 studies examined additive checkpoint inhibitor therapy in the adjuvant and neoadjuvant settings, the NeoTRIP study focused entirely on neoadjuvant atezolizumab administration [7,8]. Recently published results showed, among other things, significantly higher response rates with pembrolizumab therapy. An effect that was even more evident among nodal metastatic tumors. Although the data are not yet mature enough to assess recurrence rates, promising trends are emerging. Carey emphasized that the potential benefit of additional immunotherapy must always be considered in relation to the higher rate of serious side effects. In addition, the chemotherapy administered was potentially decisive for the additional benefit. In the analysis of various studies, the impression emerges that parallel anthracycline therapy is particularly beneficial for the effect of the checkpoint inhibitor.
Regarding PD-L1 expression, no predictive benefit for immunotherapy was observed in previous studies. PD-L1 positive tumors responded better to both checkpoint inhibitor therapy and chemotherapy alone and thus had a better prognosis in principle. Thus, PD-L1 is currently interpreted more as a general biomarker of carcinoma therapy sensitivity and may in the future serve as a marker for highly sensitive tumors for which the current regimen may be de-escalated for treatment.
Carey mentioned the number of tumor-infiltrating lymphocytes (TILs) as another prognostic marker that could gain importance in the future. This is easy to determine and has a major impact on prognosis, especially in the presence of lymph node metastases. If many TILs can be detected, the risk of recurrence is lower. Carey explained this in terms of more pronounced immune activation leading to more tumor-infiltrating lymphocytes.
Both further investigation of the potential of immunotherapy and research into the significance of various biomarkers will shape the management of triple-negative breast carcinoma in the near future. The aim is not only to improve the outcome, but also to assess the risk and thus to better adapt the therapy to the respective situation.
Source: San Antonio Breast Cancer Symposium Dec. 8-11, 2020, ES4 Educational Session “Triple Negative Breast Cancer,” Lisa Carey (University of North Carolina Lineberger Comprehensive Cancer Center).
Further reading:
- Golshan M, et al: Impact of neoadjuvant chemotherapy in stage II-III triple negative breast cancer on eligibility for breast-conserving surgery and breast conservation rates: surgical results from CALGB 40603 (Alliance). Ann Surg 2015; 262(3): 434-439; discussion 8-9.
- EBCTCG: Increasing the dose intensity of chemotherapy by more frequent administration or sequential scheduling: a patient-level meta-analysis of 37,298 women with early breast cancer in 26 randomised trials. Lancet 2019; 393(10179): 1440-1452.
- Sikov WM, et al: Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 2015; 33(1): 13-21.
- Loibl S, et al: Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol 2018; 19(4): 497-509.
- Masuda N, et al: Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017; 376(22): 2147-2159.
- Blum JL, et al: Anthracyclines in Early Breast Cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol 2017; 35(23): 2647-2655.
- Schmid P, et al: Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med 2020; 382(9): 810-821.
- Mittendorf EA, et al: Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. Lancet 2020; 396(10257): 1090-1100.
InFo ONCOLOGY & HEMATOLOGY 2021; 9(1): 28-30 (published 2/22/21, ahead of print).