Patients with Crohn’s disease can be effectively placed in clinical remission with the combination of infliximab and immunosuppressants. However, long-term use poses some dangers. Scientists from Liege have therefore investigated how discontinuation of one of the components affects remission.
Once remission is achieved with the combination of infliximab + immunosuppressant (thiopurine or methotrexate), physicians and patients must weigh the risks and benefits of continued combination therapy, write Prof. Edouard Louis, M.D., Department of Gastroenterology, University Hospital of Liège (D), and colleagues. The risk of opportunistic or serious infections and lymphoproliferative disorders is of particular concern, he said, with evidence that patients on combination therapy are at greater risk than those receiving monotherapy.
Discontinuation of infliximab in patients with Crohn’s disease in sustained remission has been associated in uncontrolled studies with an increased risk of relapse of approximately 50% over a 2-year period, although remission can be regained in most patients by resumption of infliximab treatment-particularly if immunosuppressive therapy has been continued. Other uncontrolled data suggest that discontinuation of immunosuppressive therapy does not alter relapse rates in patients who had achieved durable remission on combination therapy. In their study [1], the Belgian researchers now investigated the effects of therapy de-escalation in terms of relapse rate and duration of remission over a period of two years.
Infliximab withdrawal leads to increased recurrences
Adult patients with Crohn’s disease (n=207) who had been in clinical remission for more than 6 months and had received combination therapy of infliximab and immunosuppressants for at least 8 months were randomly assigned (1:1:1) to either continuation of combination therapy (n=67), discontinuation of infliximab (n=71), or discontinuation of immunosuppressant therapy (n=69). Over a 2-year observation period, 8 patients in the combination group (12%) relapsed, compared with 25 (35%) in the infliximab withdrawal group and 6 (9%) in the immunosuppressant withdrawal group.
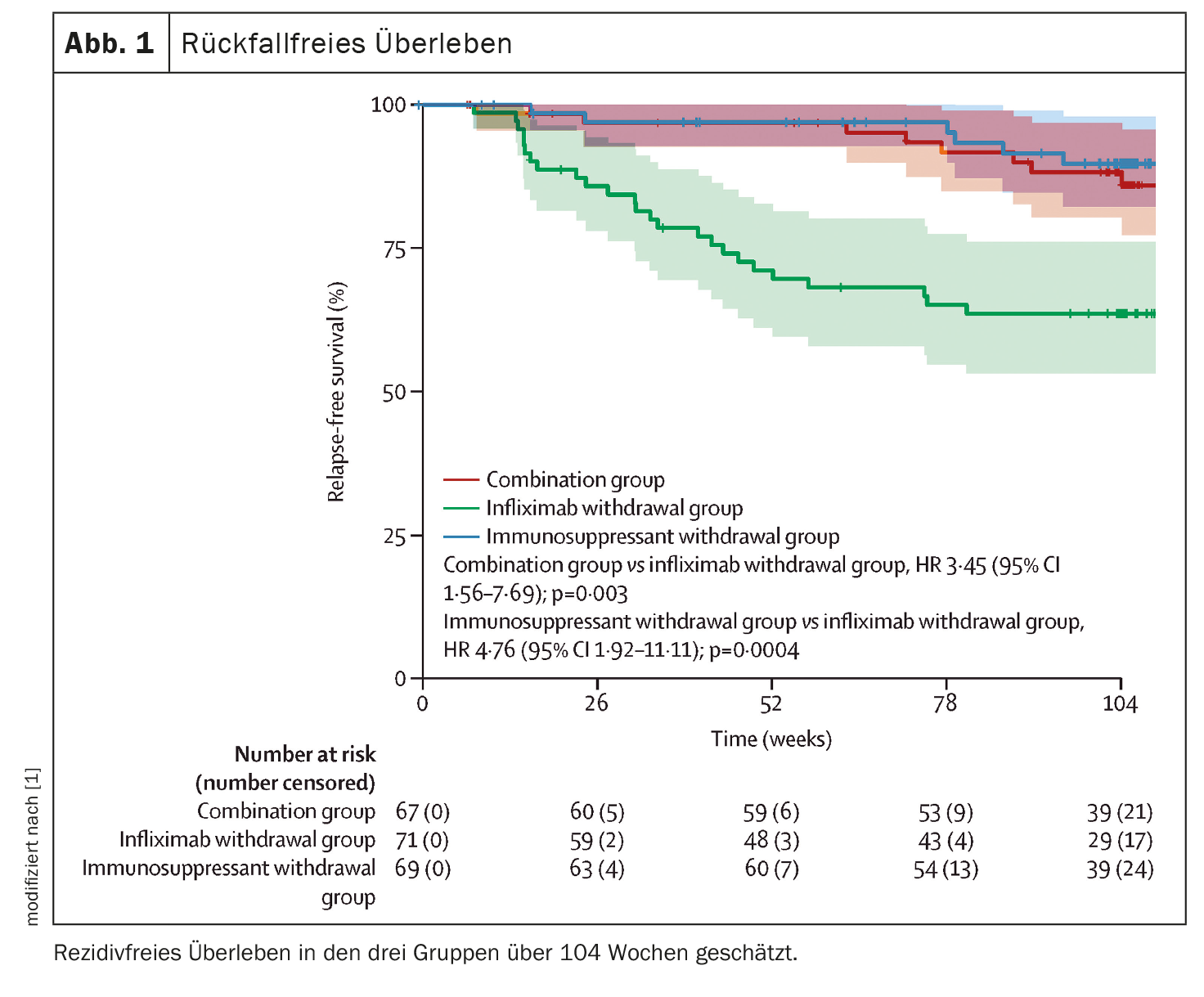
The 2-year relapse rates were 14% (95% CI 4-23) in the combination group, 36% (95% CI 24-47) in the infliximab withdrawal group, and 10% (95% CI 2-18) in the immunosuppressant withdrawal group. The corresponding HRs for relapse-free survival were 3.45 (95% CI 1.56-7.69) for the combination group versus the infliximab withdrawal group (p=0.003) and 4.76 (1.92-11.11) for the immunosuppressant withdrawal group versus the infliximab withdrawal group (p=0.0004) (Fig. 1) .
The most common serious adverse events were infections (four in the combination group, two in the infliximab withdrawal group, and one in the immunosuppressant withdrawal group) and Crohn’s disease exacerbation (three, four, and one, respectively). No deaths or malignancies were recorded.
The median duration of remission was 698 days (95% CI 668-727) in the combination group, reduced to 684 days (95% CI 651-717) in the infliximab withdrawal group, and 706 days (95% CI 682-730) in the immunosuppressant withdrawal group. The difference in restricted median survival in remission was -14 days (95% CI -56-27) between the infliximab withdrawal group and the combination group and -22 days (95% CI -62-16) between the infliximab withdrawal group and the immunosuppressant withdrawal group. The 95% CIs both included the threshold for noninferiority of -35 days.
The study showed an increased risk of relapse over two years in patients undergoing infliximab withdrawal compared with those patients who continued infliximab either as monotherapy or in combination with an immunosuppressant. Conversely, discontinuation of immunosuppressive therapy had no effect on the relapse rate. Re-treatment with infliximab allowed rapid recovery and maintenance of remission in patients who relapsed, and rates of treatment failure were similar in all treatment groups. Despite this result, the hypothesis of noninferiority in terms of duration of remission over two years after infliximab discontinuation was rejected.
Sustained clinical remission without steroids over two years did not differ significantly among the three groups. The main reasons for not achieving sustained clinical remission without steroids were early withdrawal and a fluctuating CDAI** score of 150 or higher, which were not systematically associated with confirmed relapse. The lack of a difference in relapse rates between patients who continued immunosuppression therapy with infliximab and those who discontinued it confirms the lack of clinical benefit of continued immunosuppression therapy in patients treated with infliximab for a median of more than two years, Prof. Louis and his colleagues write.
** CDAI=Crohn’s Disease Activity Index
Interestingly, no acute infusion reaction was observed in the group of patients who discontinued infliximab and were treated again after a drug pause. This observation is consistent with the lack of development of anti-infliximab antibodies in this group and also with previous results.
Loss of time in remission low
In summary, the results of the study show that discontinuation of infliximab in patients receiving combination therapy is associated with an increased risk of relapse compared with patients continuing combination therapy and also with those switched to infliximab mono. In the group of patients who relapsed, the vast majority responded immediately to retreatment with infliximab, so that the loss of time in remission over two years was only 2-3 weeks.
In patients with Crohn’s disease who are in sustained steroid-free remission on combination therapy with infliximab and immunosuppressants, discontinuation of infliximab should be considered only after careful consideration of the risks and benefits for each individual patient. Discontinuation of immunosuppressants, on the other hand, may generally be a preferable strategy when treatment de-escalation is being considered, the authors conclude.
Literature:
- Louis E, Resche-Rigon M, Lahari D, et al: Withdrawal of infliximab or concomitant immunosuppressant therapy in patients with Crohn’s disease on combination therapy (SPARE): a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol 2023; 8: 215-227; doi: 10.1016/S2468-1253(22)00385-5.
GASTROENTEROLOGY PRACTICE 2023; 1(1): 32-33