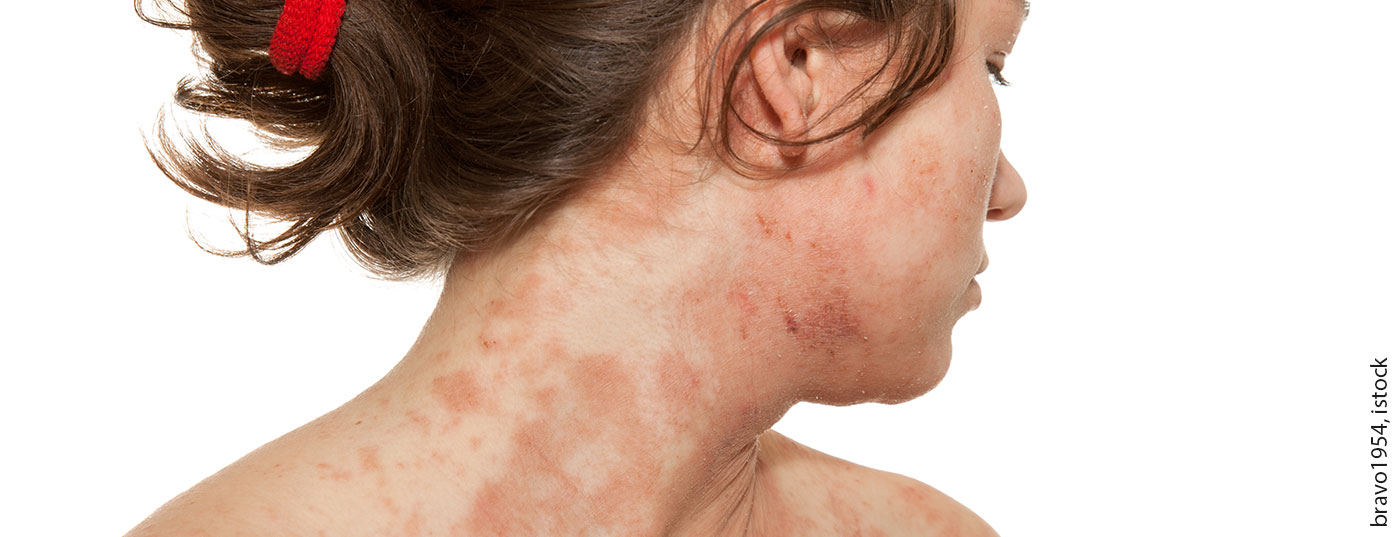
When topical steroid therapy alone is not sufficiently effective, patients with atopic dermatitis may benefit greatly from the additional use of the JAK inhibitor baricitinib. This is shown, among other things, by a recent analysis of data from the BREEZE-AD7 study, which focused on improvements from the patient’s perspective (“patient-reported outcomes”).
The oral selective Janus kinase (JAK)-1/2 inhibitor baricitinib (Olumiant®) was approved in Switzerland in February 2021 for the treatment of adult patients with moderate to severe atopic dermatitis [1]. In several randomized-controlled trials, including BREEZE-AD1 and BREEZE-AD2, baricitinib monotherapy resulted in significant symptom reduction in moderate-to-severe atopic dermatitis [2]. The phase III BREEZE-AD7 trial was conducted to evaluate the efficacy and safety of baricitinib in combination with topical corticosteroids (TCS) in adults who had responded inadequately to TCS treatment alone [3]. 329 adult patients with moderate-to-severe atopic dermatitis were randomized 1:1:1 to baricitinib 4 mg plus TCS or baricitinib 2 mg plus TCS or placebo. Treatment was daily in each of the study arms. That baricitinib combined with topical steroids resulted in significant levels of objectively assessable symptom reduction (e.g., pruritus NRS or EASI75 response) has already been reported in other publications of the BREEZE-AD7 trial data [4]. In a recent publication in the Journal of the European Academy of Dermatology and Venereology by Wollenberg et al. the benefit of the combination therapy of baricitinib plus TCS was analyzed from the patient’s perspective [3].
“Patient-reported outcomes” demonstrate rapid and sustained improvements
During 16 weeks, treatment effects on various patient-reported outcomes were recorded [3]. Patient-reported outcomes are increasingly important because they represent an assessment of the burden of disease and the effectiveness of treatment from the patient’s perspective. Therapy with baricitinib 4 mg (n=111) or 2 mg (n=109) plus TCS resulted in rapid, statistically significant improvements in this regard compared with placebo (n=109). As early as week 2, baricitinib combined with TCS showed a ≥4-point improvement in the Dermatology Life Quality Index (DLQI) score (p≤0.001 and p≤0.05, respectively) and, at the higher dose, a significantly superior response in terms of pruritus impairment, operationalized using the Patient-Reported Outcome Measurement Information System (PROMIS) itch scale. The PROMIS Itch Short Form measures the extent to which itch limited health-related quality of life (HRQoL) in the past 7 days [5].

At week 16, both doses of baricitinib plus TCS showed significantly improved Patient Benefit Index (PBI) global scores compared with placebo (p≤0.001 and p≤0.05, respectively) (Fig. 1) [3]. Moreover, patients treated with baricitinib 4 mg plus TCS also scored significantly better on the PBI subscales of social limitations and psychological impairment compared with placebo. In the PBI, subjectively perceived treatment effects are rated on a scale from 0 “not at all helpful” to 4 “very helpful.” Multifactorial analysis of variance was performed for statistical comparisons.
Source: Eli Lilly
Literature:
- Drug information, www.swissmedicinfo.ch, last accessed 02.12.2021
- Melo A, Carrascosa JM, Torres T: Baricitinib for the treatment of atopic dermatitis. J Dermatolog Treat 2021 Aug 23: 1-10. Epub ahead of print.
- Wollenberg A, et al: Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. JEADV 2021; 35(7): 1543-1552.
- Reich K, et al: Efficacy and Safety of Baricitinib Combined With Topical Corticosteroids for Treatment of Moderate to Severe Atopic Dermatitis: A Randomized Clinical Trial. JAMA Dermatol; 156(12): 1333-1343.
- Silverberg JI, et al: Development, validation, and interpretation of the PROMIS itch questionnaire: a patient-reported outcome measure for the quality of life impact of itch. J Invest Dermatol 2020; 140: 986-994.e986.
DERMATOLOGY PRACTICE 2021; 31(6): 38