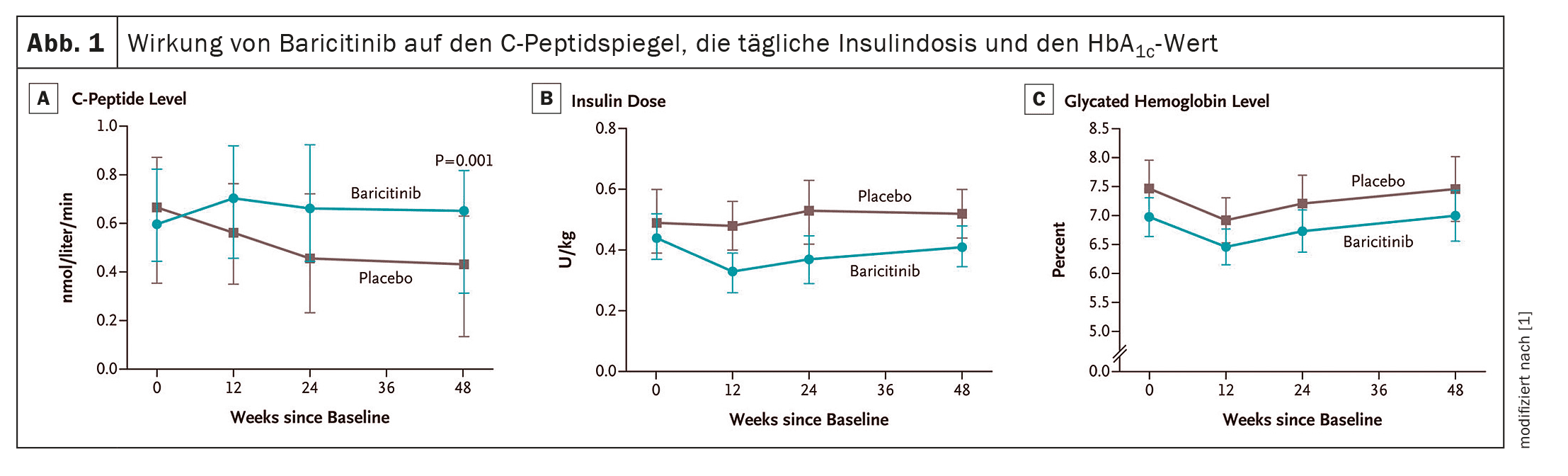
Baricitinib, a JAK1 and JAK2 inhibitor, is often used to treat rheumatoid arthritis, alopecia areata or severe coronavirus disease. Doctors in Australia have also investigated whether the drug could preserve the function of β-cells and improve metabolic values in patients with new-onset type 1 diabetes.
Dr. Michaela Waibel from St. Vincent’s Institute of Medical Research (SVI) in Melbourne, Australia, and colleagues conducted a double-blind, randomized, placebo-controlled phase 2 study in which patients with type 1 diabetes diagnosed within the previous 100 days received baricitinib (4 mg orally once daily) or placebo for 48 weeks [1]. The primary endpoint was the mean C-peptide level as measured by AUC during a 2-hour mixed-meal tolerance test. Secondary endpoints included changes in HbA1c and daily insulin dose as well as glucose control using continuous glucose monitoring (CGM).
Advantages of C-peptide
A total of 91 patients received baricitinib (n=60) or placebo (n=31). The results showed clear benefits in terms of the primary endpoint for those patients who received baricitinib: The median of the mean C-peptide level stimulated by a mixed meal at week 48 in this group was 0.65 nmol per liter per minute – in the controls this value was only 0.43 nmol (p=0.001). The mean daily insulin dose after 48 weeks was 0.41 U per kilogram of body weight per day (95% CI 0.35-0.48) in the baricitinib group and 0.52 U (95% CI 0.44-0.60) in the placebo group. The glycosylated hemoglobin values were similar in the two study groups (Fig. 1). However, the mean coefficient of variation of glucose levels at 48 weeks, as measured by CGM, was 29.6% (95% CI 27.8-31.3) in the baricitinib group vs. 33.8% (95% CI 31.5-36.2) in the placebo group. The frequency and severity of adverse events were similar in the two study groups, and no serious adverse events were attributed to baricitinib or placebo.
Dr. Waibel and colleagues found evidence that preserving residual β-cell function after the diagnosis of type 1 diabetes reduces the need for exogenous insulin and is associated with protection against vascular complications and severe hypoglycemia.
Autoreactive CD8+ T cells in type 1 diabetes bind to an autoantigen peptide bound to HLA class I molecules on the surface of β-cells and are thereby activated, leading to the release of perforin and granzymes that cause β-cell death. The authors were able to show that the interaction between CD8+ T cells and HLA class I molecules requires Janus kinase (JAK)-associated intracellular signaling molecules. Inhibitors of JAK1 and JAK2 isoforms impair cytokine-induced major histocompatibility complex class I expression in cultured islets and islet cells, impair CD8+ T cell activation, and block immune synapse formation between β-cells and CD8+ T cells to prevent β-cell death. In addition, activating mutations in the signaling molecules STAT1 and STAT3, which are downstream of JAKs, are associated with the development of autoimmune diabetes.
Baricitinib maintains the ability of β-cells to secrete insulin
The magnitude of the effect at 48 weeks – a 48% increase in median mixed meal-stimulated mean C-peptide levels in the baricitinib group vs. placebo – was comparable to the magnitude of the effect of interventions such as teplizumab, low-dose antithymocyte globulin and golimumab, which are currently considered the most effective disease-modifying therapies in patients with T1D, the researchers write. In contrast to these agents, which require i.v. infusion or s.c. injection, the effect of baricitinib was achieved through the daily intake of a single tablet.
The results of their study indicate that baricitinib improved the readings obtained using CGM. The results support the use of continuous glucose monitoring to assess the outcomes of immunotherapy in patients with T1D. Patients in the baricitinib group generally required a lower daily insulin dose, although all but three of these patients required exogenous insulin therapy at the end of the treatment period.
Dr. Waibel’s team assumes that in many patients with stage 3 type 1 diabetes, the β-cell mass is already too irreversibly damaged at the time of diagnosis for cessation of insulin therapy to be a realistic goal. The median of the mean C-peptide level stimulated by a mixed meal at the time of screening in this study was 0.7 nmol per liter. It can be speculated that starting treatment with baricitinib earlier, when C-peptide levels are higher, may be more effective in reducing the need for injected insulin.
The Australian researchers recommend that other JAK inhibitors should also be tested for their effect on type 1 diabetes.
Literature:
- Waibel M, et al.: N Engl J Med 2023; 389(23): 2140–2150; doi: 10.1056/NEJMoa2306691.
InFo DIABETOLOGIE ENDOKRINOLOGIE 2024; 1(3): 40