Treatment of elevated LDL cholesterol levels is a central component of modern therapy to reduce the risk of serious cardiovascular events in patients with primary or secondary prevention. However, 7% to 29% of patients report muscular side effects that prevent them from taking statins or increasing the dose to meet guidelines. A recent study published in NEJM suggests alternative strategies.
This avoidance of statin therapy significantly increases the risk of cardiovascular events. Bempedoic acid, an ATP citrate lyase inhibitor, offers an alternative method of lowering LDL cholesterol and appears to be associated with a lower incidence of muscle-related adverse events. The aim of the study discussed was to investigate the effects of bempedoic acid on cardiovascular events in statin-intolerant patients.
Methods
Study design: This double-blind, randomized, placebo-controlled study included 13,970 patients who were either unable or unwilling to take statins due to unwanted side effects. The patients were randomly divided into two groups: One group received 180 mg of oral bempedoic acid daily, the other a placebo. The primary endpoint was a four-part composite of major cardiovascular events, including death from cardiovascular causes, non-fatal myocardial infarction, non-fatal stroke or coronary revascularization.
Participants: The study included patients aged 18 to 85 years who had either had a cardiovascular event in the past (secondary prevention) or had clinical features that put them at high risk of a cardiovascular event (primary prevention). All participants had to confirm in writing that they were statin intolerant and aware of the benefits of statins in reducing cardiovascular risk.
Randomization and treatment regimen: Patients initially underwent a 4-week run-in phase with placebo. Upon successful completion, they were randomized in a 1:1 ratio to receive either bempedoic acid or placebo. Central laboratories monitored LDL cholesterol levels and notified study physicians of levels 25% or more above baseline to take appropriate action.
Results
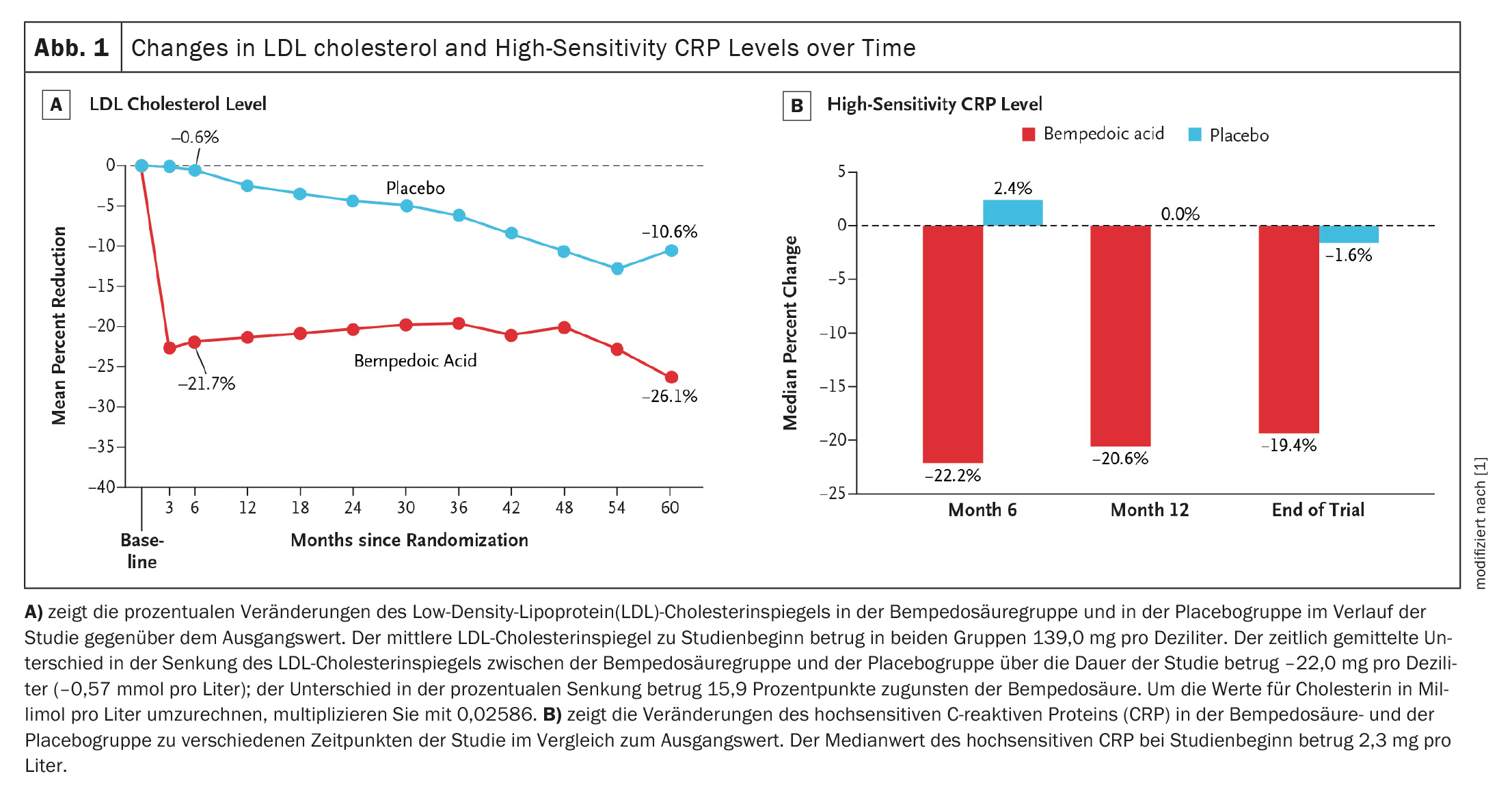
Baseline data and follow-up: Of the 13,970 randomized patients, 6992 received bempedoic acid and 6978 received placebo. The median follow-up time was 40.6 months. The mean LDL cholesterol level at baseline was 139.0 mg/dl in both groups. After six months, the reduction in LDL cholesterol levels was 29.2 mg/dl greater in the bempedoic acid group than in the placebo group, which corresponds to a percentage difference of 21.1 percentage points.
Primary and secondary endpoints: The incidence of a primary endpoint event was significantly lower in the bempedoic acid group than in the placebo group (11.7% vs. 13.3%; hazard ratio, 0.87; 95% CI, 0.79 to 0.96; p=0.004). The incidences of secondary endpoints such as a composite endpoint of death from cardiovascular causes, non-fatal stroke or non-fatal myocardial infarction (8.2% vs. 9.5%; hazard ratio, 0.85; 95% CI, 0.76 to 0.96; p=0.006), non-fatal myocardial infarction (3.7% vs. 4.8%; hazard ratio, 0.77; 95% CI, 0.66 to 0.91; p=0.002) and coronary revascularization (6.2% vs. 7.6%; hazard ratio, 0.81; 95% CI, 0.72 to 0.92; p=0.001) were significantly lower in the bempedoic acid group.
Side effects: The overall incidence of side effects, severe side effects and side effects that led to discontinuation of therapy did not differ significantly between the groups.
However, the incidences of gout (3.1% vs. 2.1%) and cholelithiasis (2.2% vs. 1.2%) were higher in the bempedoic acid group.
Slight increases in serum creatinine, uric acid and liver enzymes were also observed more frequently.this study shows that bempedoic acid significantly reduces the risk of serious cardiovascular events in statin-intolerant patients.
The reduction in LDL cholesterol by 22.0 mg/dl over the course of the study corresponds to an approximately 13% lower incidence of the primary endpoint.
These results are consistent with the meta-analytic prediction of the Cholesterol Treatment Trialists’ Collaboration and the effects of other LDL cholesterol-lowering therapies such as PCSK9 inhibitors and ezetimibe.
Comparison with other therapies: Compared with statins, which are associated with an increase in HbA1c levels and the incidence of diabetes, bempedoic acid showed no significant increase in HbA1c levels or diabetes risk.
In addition, bempedoic acid reduced hsCRP levels by 21.6% compared to placebo, indicating an anti-inflammatory effect that was not observed with the other non-statin-based LDL-lowering agents.
Conclusions
Treatment with bempedoic acid is associated with a significant reduction in the risk of serious cardiovascular events in patients who cannot or do not want to take statins due to undesirable side effects. This supports the use of bempedoic acid as an alternative therapy for LDL cholesterol lowering and cardiovascular risk reduction in statin intolerant patients.
Source:
- Nissen SE, et al.: Bempedoic Acid and Cardiovascular Outcomes in Statin-Intolerant Patients. in:New England Journal of Medicine, published on March 4, 2023, at NEJM.org. DOI: 10.1056/NEJMoa2215024.