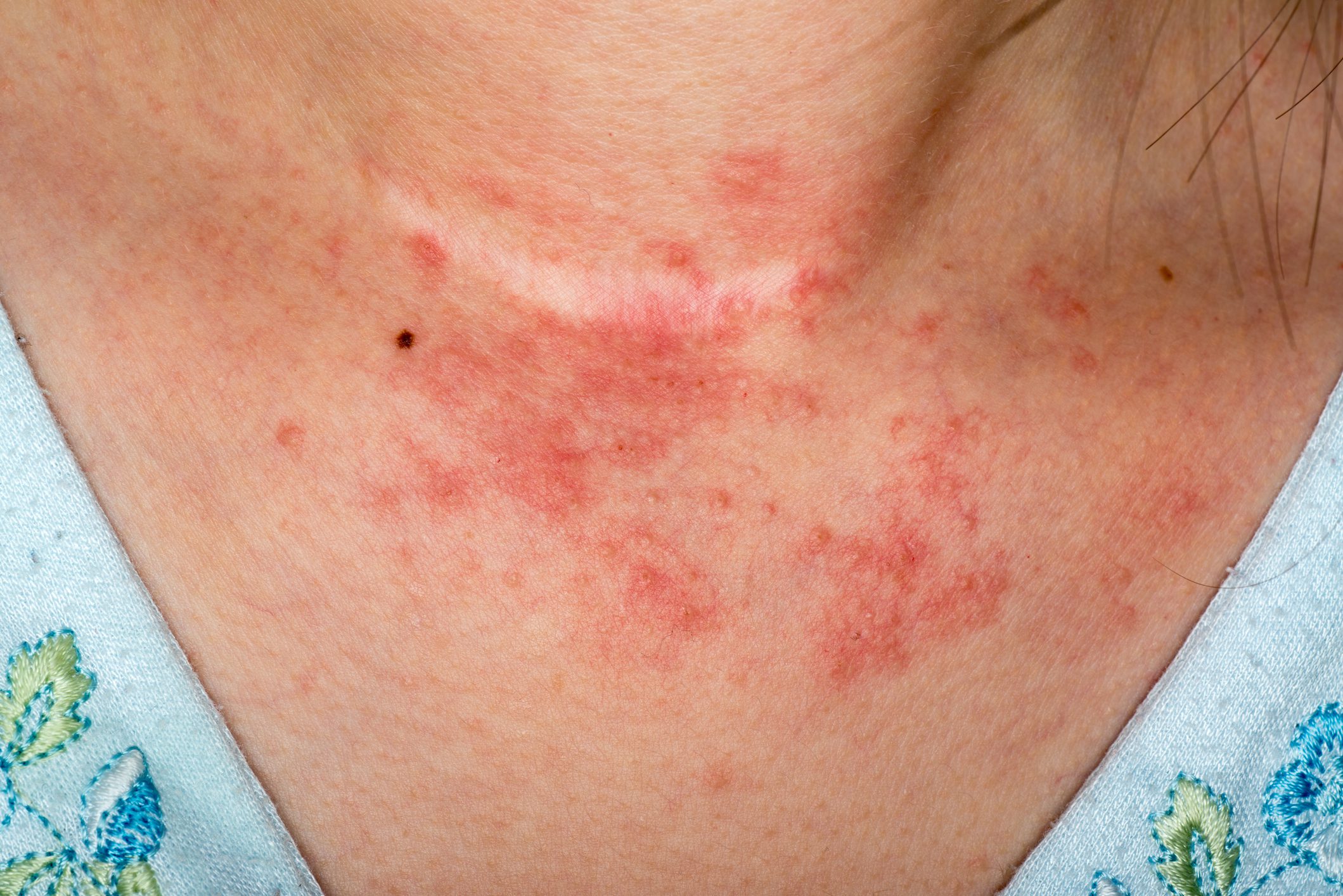
Although there have been no recent approvals of oral JAK inhibitors (JAK-i) for atopic dermatitis (AD), some interesting study findings have been published. These shed light on the safety of baricitinib, abrocitinib and upadacitinib with reference to the EMA opinion on JAK-i issued some time ago. In the EU, baricitinib has now been given an extended indication for AD patients aged 2 years and older.
Autoren
- Mirjam Peter, M.Sc.
Publikation
- DERMATOLOGIE PRAXIS
Related Topics
You May Also Like
- Case Report
76-year-old patient with pustular skin rash
- Sponsored Content: Psoriasis
Dauerhafte Erscheinungsfreiheit auch bei betroffenen speziellen Hautarealen
- Antithymocyte globulin in children with T1D
Old medicine, new hope
- Ginkgo biloba
Database of preclinical and clinical studies is becoming increasingly larger
- Digital biomarkers
Continuous monitoring using digital biomarkers in MS care
- Benefits, limits and safety aspects
Phytotherapy for cardiovascular diseases
- Results of the FOREST HCM study
Symptomatic obstructive hypertrophic cardiomyopathy
- Neuroprotection, resilience and cognitive health in old age