It is already foreseeable that biologics will also become more important in the treatment of systemic lupus erythematosus (SLE). Data were presented at the American College of Rheumatology (ACR) 2021 Annual Meeting providing further insight into the efficacy and interactions of the three agents, anifrolumab, belimumab, and rituximab [1].
Anti-interferon-α therapy with anifrolumab for systemic lupus erythematosus is already on the market in the United States. At the end of last year, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency followed suit and issued a recommendation for approval.
Anifrolumab is intended for use as an add-on therapy to standard treatment in adult patients with moderate to severe, active autoantibody-positive SLE. The antibody binds to subunit 1 of the type 1 interferon receptor and blocks not only the activity of interferon (IFN)-α but also that of other interferons such as IFN-β, IFN-ε and IFN-ω. Decisive factors for the U.S. approval included the two randomized, double-blind placebo-controlled Phase 3 studies TULIP-1 and TULIP-2 [2,3].
Anifrolumab before approval
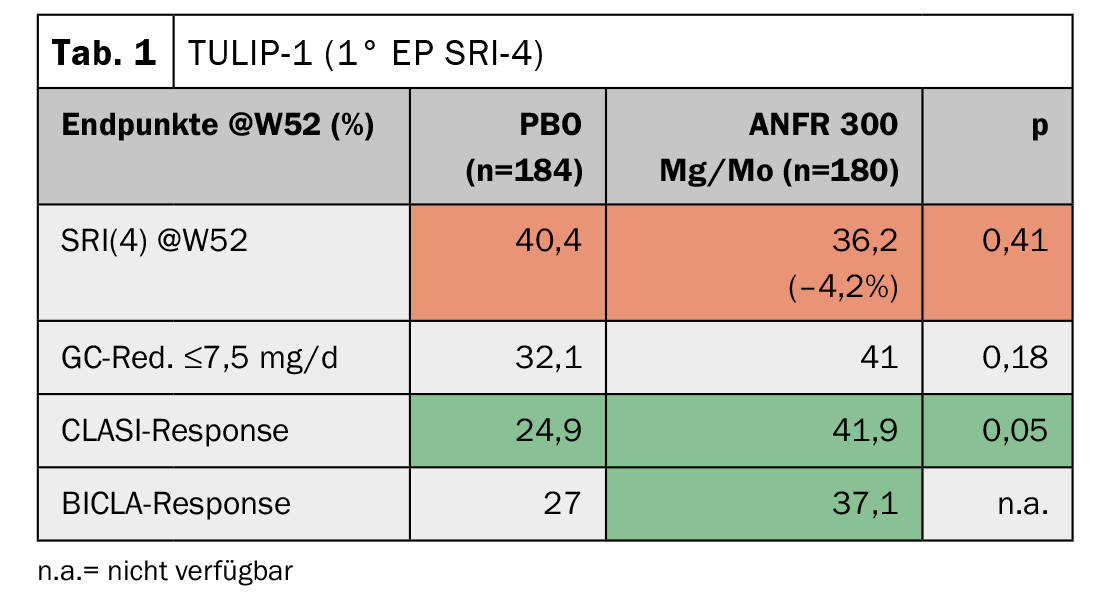
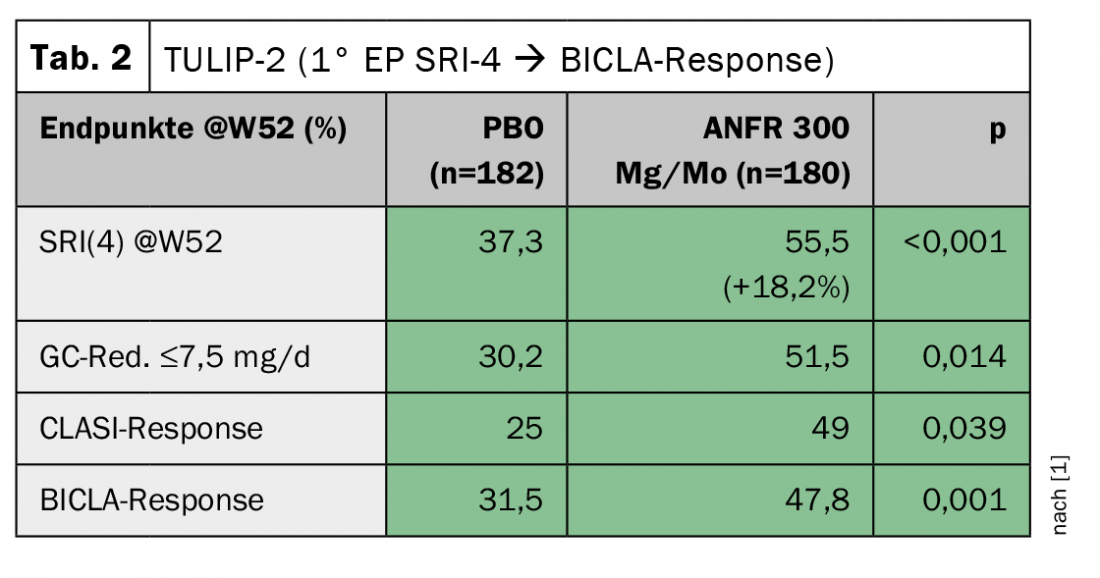
In TULIP-1, the responder index SRI(4) was chosen as the primary endpoint, as has been the case in several lupus studies previously. Here, anifrolumab even showed a slightly worse response compared to stadard therapy (Table 1) . The skin score (CLASI), however, improved significantly. The BICLA score, which is not dissimilar to SRI(4), again showed a trend toward improvement. “To this day, one cannot explain why the results differ so much,” said Professor Dr. Christof Specker, Evangelisches Krankenhaus, Kliniken Essen-Mitte (D). “The study authors then approached the FDA to switch the primary endpoint in TULIP-2 from SRI(4) to BICLA response.” Despite this change being made after the fact, the FDA accepted it. The result was indeed significant, but it also turned out that the switch would not have been necessary at all, because the SRI(4) now also showed a marked improvement here (Tab. 2). The immense difference of -4.2% vs. +18.2% in two studies conducted under the same inclusion and exclusion criteria should be reason to take another closer look at this, Prof. Specker warns. The success of the second TULIP study nevertheless led to the U.S. approval of the substance.
As already indicated by the CLASI score, patients with skin involvement seem to be predestined for therapy. In addition, at the ACR, the response to anifrolumab was scrutinized in serological subgroups. The evaluation showed that patients with low complement appeared to have a better response compared to those who did not. However, this was also the only subgroup of the serological type in which a difference could be worked out, Prof. Specker pointed out. Furthermore, the clinical relevance in a real-life setting cannot yet be well assessed.
The safety profile proved favorable, as indicated by evaluations both on the ACR and previously on the EULAR. Only an increased rate of zoster effects was noticeable. The expert’s advice was therefore to strongly recommend herpes zoster vaccination to those patients who are to receive anifrolumab in the future.
|
COVID-19 and SLE What is the relationship between lupus disease and the severity of the course in covid 19 infection? An evaluation of 1734 SLE patients showed that the greatest risk factor for severe COVID-19 progression was high disease activity, followed by renal insufficiency and prednisone doses ≥5 mg/d [5]. However, no lupus therapy is not the answer either, because those patients still have twice the risk of those with stable, low disease activity. Over 90% of all SLE patients tolerate vaccination well [7]. When “flares” did occur, they proved to be brief, mild, and virtually indistinguishable from normal vaccination reactions. Prof. Specker concludes that patients can be advised to vaccinate with a clear conscience. |
Belimumab vs. rituximab
According to Prof. Specker, rituximab (RTX) is still the biologic most commonly used for lupus nephritis – whereas belimumab (BEL) is the agent that also has approval, recently as an add-on therapy for lupus nephritis. The BLISS-BELIEVE study [4] presented at the ACR as a late-breaking abstract compared belimumab and rituximab consecutively. 396 patients were screened for this, but only 292 were ultimately randomized into the study and treated. 92% of participants were female with an average age of 40.5 years (+/- 12 years). Compared with baseline, patients had a SLEDAI score of 10.3.
Divided into three groups, BEL and RTX were compared with the effect of placebo (PBO) and standard of care (SOC). Patients with active SLE received treatment for 52 weeks:
- Group 1: BEL s.c. + PBO at weeks 4 and 6 (n=72).
- Group 2: BEL s.c. + RTX at weeks 4 and 6 (n=144).
- Group 3: BEL + SOC (n=76)
The treatment was followed by a 52-week observation phase. The primary endpoint was a SLEDAI-2K activity score of ≤2, with no use of other immunosuppressants and cortisone administration of ≤5 mg at week 52. Discontinuation rates were relatively high at approximately 19% in all groups. There were no significant differences in disease control for BEL/RTX vs. BEL/PBO.
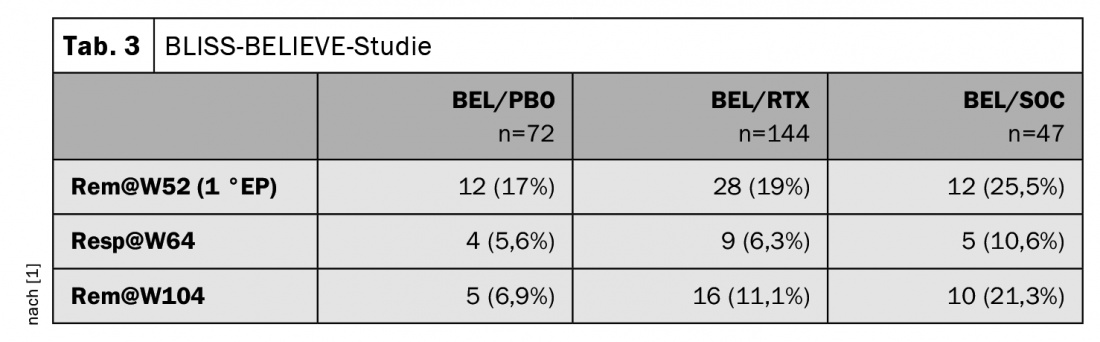
There were no significant differences in disease control for BEL/RTX vs. BEL/PBO; however, a comparison of BEL/PBO vs. BEL/SCO was not made as part of the study. This was surprising to Prof. Specker, since there were clear differences in remission and response between the BEL/PBO and BEL/RTX groups and the BEL/SOC group: the standard-of-care treatment was consistently and significantly better ( Table 3). However, the case numbers were not very high, he noted caveatorily. With regard to the full paper, the expert is therefore curious to see whether the differences may already reach significance.
In terms of adverse events, there were slightly more serious AEs under BEL/RTX, which were mostly in the form of infections. However, the favoring of infections by the administration of RTX – especially in the initial phase of treatment – is well known.
Sources:
- Streamedup: Post ACR 2021, 09.12.2021.
- Furie R, et al: Lancet Rheumatology 2019; 1(4): e208-e219.
- Morand EF, et al: New England Journal of Medicine 2020; 382(3): 211-221.
- Aranow C, et al: ACR 2021 [#L 13]; Arthritis Rheumatol 2021; 73 (suppl 10).
- Ugarte-Gil M, et al: ACR 2021 [#0866]; Arthritis Rheumatol 2021; 73 (suppl 10).
- Petri M, et al: ACR 2021 [#0858].
- Barbhaiya M, et al: ACR 2021 [#0896].
InFo PAIN & GERIATry 2022; 4(1-2): 35-36.
HAUSARZT PRAXIS 2022; 17(8): 39-40











