Botulinum toxin (BTX) has evolved from a former ophthalmic drug with a limited therapeutic spectrum to a drug with worldwide distribution. It has a presynaptic inhibitory effect on the release of ACh at the neuromuscular endplate on the one hand and at the neurovegetative endplate on the other. In medical therapeutic application in humans, practically only BTX A is used, and there are three preparations that have been tested in a large number of scientific studies: Onabotulinum, Abobotulinum, Incobotulinum. How are they used and what material is needed for botulinum treatment?
The first recorded case of botulinum toxin poisoning with the neurotoxin-producing bacterium Clostridium botulinum dates back to 1735, when the band playing at a wedding party was fed spoiled ham and smoked sausages. Four of the ten musicians died of botulism [1]. Dr. Justinus Christian Kerner (1786-1862), poet and medical officer of Baden-Württemberg (Fig. 1), published in the “Tübinger Blättern für Naturwissenschaften und Arzneykunde” meticulously the symptoms of patients suffering from botulism and already noted at that time that “this toxin could probably be a formidable medicine for the treatment of spasms, as well as for the reduction of excessive salivation, lacrimation or perspiration” at a later time [2]. Since the bacterium had not yet been discovered at that time, Kerner believed in the so-called fatty acid theory after numerous other mechanisms had been discussed at length (unhealthy pig feed, electrical energy from lightning strikes, God’s punishments due to dishonest lifestyles, etc.). During this time, botulism was also called Kerner’s disease.

In 1840, it was Friedrich Gustav Jakob Henle (1809-1885), an anatomy professor from Göttingen (“Henle’s loop”), who first described parasitic organisms (Contagium vivum) as possibly causing disease. 20 years later, the chemist Louis Pasteur (1822-1895) demonstrated the microbial fermentation of wine. In 1882, Robert Koch (1843-1910), a student of Henle, discovered the tubercle bacillus and was awarded the Nobel Prize for it in 1905. The consequences that resulted from the discovery of bacteria were enormous. For the first time in the history of medicine, the causes of numerous diseases became known, making causal therapy possible for the first time – at least to some extent at the beginning. In 1897, Pierre Marie van Ermengem (1851-1932), a student of Robert Koch, was able to isolate the pathogen responsible for botulism, thus disproving Kerner’s fatty acid theory. He named this bacterium Bacillus botulinus, which is now known as Clostridium botulinum [3]. In 1905, Tchitchikane identified the neurotoxin. On behalf of the U.S. Army, Eduard J. Schantz had been studying the structure of the protein since World War II, when it became known that various countries had capabilities and plans to use chemical and biological weapons. His research on protein structure and mechanism of action laid the foundation for the current use of the toxin as a drug. In 1949, Burgen’s studies were to show that the effect of the toxin was due to presynaptic acetylcholine inhibition (chemodenervation) and not, as previously thought, to postsynaptic nerve blockade [4]. This finding led the way for later theoretical foundations for the clinical application of the toxin (see below).
In 1973, Dr. Alan Scott pioneered the clinical use of botulinum toxin (BTX) by using BTX for the first time in primates to treat strabismus. He worked closely with E. J. Schantz, who was responsible for the production of the toxin. However, if the toxin was to be used therapeutically as a drug in humans, it first had to be purified from other proteins to prevent antibody formation as efficiently as possible and to ensure stability at high dilution. The problems were solved and eight years after the first animal experiment in monkeys, Scott was able to publish the first application of BTX in humans under the title “Botulinum toxin injection of eye muscles to correct strabismus” [5].
20 years after the clinical development of the drug, BTX was approved by the U.S. FDA for the treatment of strabismus, blepharospasm and hemifacial spasm. Rather accidentally, Jean Carruthers, a resident with Dr. Scott, discovered that the wrinkles on the treated side of patients with hemifacial blepharospasm smoothed out significantly [6]. At about the same time, Bushara discovered a significant reduction in sweating in the treated half of the face and published for the first time a possible treatment of axillary hyperhidrosis with BTX [7]. These two findings were the beginning of a success story of a former ophthalmic drug with limited therapeutic use, which became a drug with worldwide distribution. In Switzerland, BTX was first used for the treatment of focal hyperhidrosis in 1997. The first hyperhidrosis consultation in Switzerland was established at the University Hospital in Zurich [8]. Over the years, aesthetic treatment indications have become increasingly important. According to statistics from the American Society for Aesthetic Plastic Surgery, 3,766,148 aesthetic botulinum treatments were performed in the U.S. alone in 2013 – not including treatment for hyperhidrosis. This represents an increase of 5680% compared to 1997!
All Botox® or what?
To date, seven immunologically distinct types of BTX are known (A-G), with toxins A, B, E, and F showing efficacy in humans, whereas toxins C and D cause disease only in animals, and toxin G has not yet been described to cause disease in either humans or animals [9]. In medical therapeutic applications, BTX A is used almost exclusively. Botulinum toxin B complex, marketed as the brand name Myobloc® in Europe and Neurobloc® in the USA (Elan Pharmaceuticals, USA), plays only a marginal role in dermatology due to limited duration of action and burning pain on injection.
There are three main BTX A products that have been tested in a variety of scientific preclinical and clinical studies:
- Onabotulinum toxin A: Botox®/Vistabel® (Actavis/Allergan, USA)
- Abobotulinum toxin A: Dysport®/Azzalure® (Ipsen, UK resp. Galderma, CH)
- Incobotulinum Toxin A: Xeomin®/Bocouture® (Merz Aesthetics, D)
Botox®, Dysport® and Xeomin® are used for medical indications (neurological disease patterns/hyperhidrosis), Vistabel® (or also Botox aesthetics® in the USA), Azzalure® and Bocouture® are available for the aesthetic indications, whereby the respective sibling preparations are pharmacologically absolutely identical and they only differ in the brand name for better identification (FDA requirement). Not every preparation mentioned has the approval for every indication. However, this circumstance is country-specific and is therefore not discussed in more detail here.
The individual toxins (Onabotulinum, Abobotulinum, Incobotulinum) differ in terms of their dosage form (units/package), transport conditions and biological activity, which is shown in Table 1.
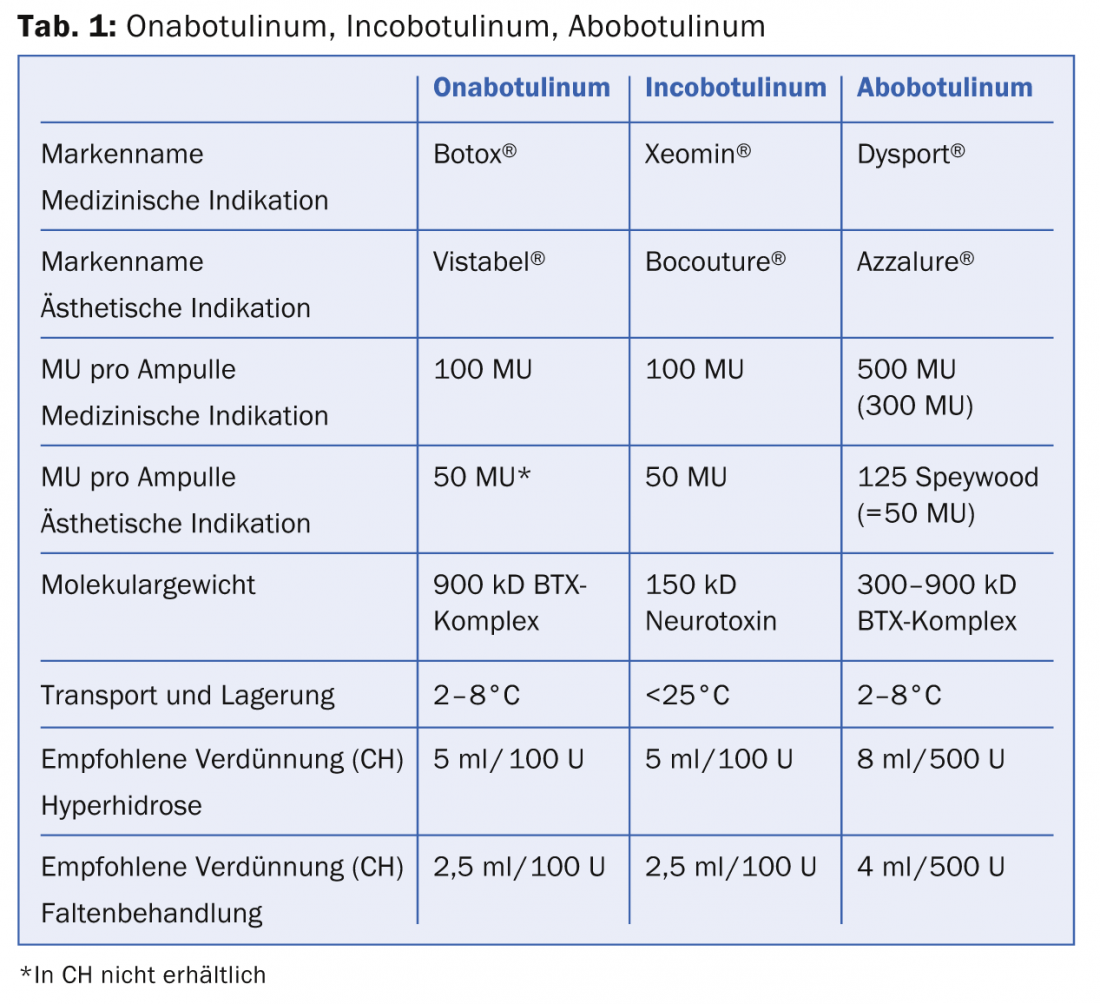
All three products come in a crystalline dry substance and must be reconstituted with 0.9% NaCl. While Ona- and Abobotulinum must be transported and stored refrigerated, this cold chain is not required for Incobotulinum. However, after reconstitution, all products should be stored in a cool place. The biological activity of all three products is expressed in so-called mouse units (MU). 1 MU is equivalent to 0.2X10-12 mol (picomoles) of toxin and is defined as the amount of toxin that is lethal to half the population of female Swiss Webster Mice (18-22 g) after intraperitoneal injection (Lethal Dose LD50). Since June 2011, Allergan has been the only competitor to perform an in vitro cell-based assay (CBPA, “cell based potency assay”) to determine the stability and efficacy of the drug. Onabotulinum and incobotulinum are comparable in terms of efficacy, duration of action and onset of action. Incobotulinum is the only complex protein-free preparation, which is said to be characterized by a reduced neutralizing antibody response (prevention of secondary treatment failure). It is important to note that the equivalent dose for abobotulinum (Dysport® resp. Azzalure®) is not the same as the dose of the other two BTX (Tab. 1). This is due to the fact that different bacterial strains are used to produce the drug and the manufacturing processes are different. The dose conversion factor between onabotulinum/incobotulinum to abobotulinum is 1:3 (resp. 1:2.5 depending on the publication). This is of utmost importance when reconstituting the toxin in clinical use to avoid over- or underdosing. More for the sake of completeness, it should be mentioned that the World Wide Web offers a variety of BTX products with different brand names of uncertain origin. It goes without saying that these products should be avoided.
How does botulinum work?
BTX has a presynaptic inhibitory effect on the release of ACh at both the neuromuscular and neurovegetative endplates, as mentioned above. In other words, both the muscle and the autonomic nervous system (VNS) are served by the same transmitter substance (ACh). That is why BTX is effective both in muscle and in other vegetatively controlled processes (hyperhidrosis, hypersalivation, urological indications, etc.) (Fig. 2). And this extremely specific mechanism of action of BTX is also the reason why BTX has no side effect in clinical use. ACh is used as transmitter exclusively in the mentioned systems.
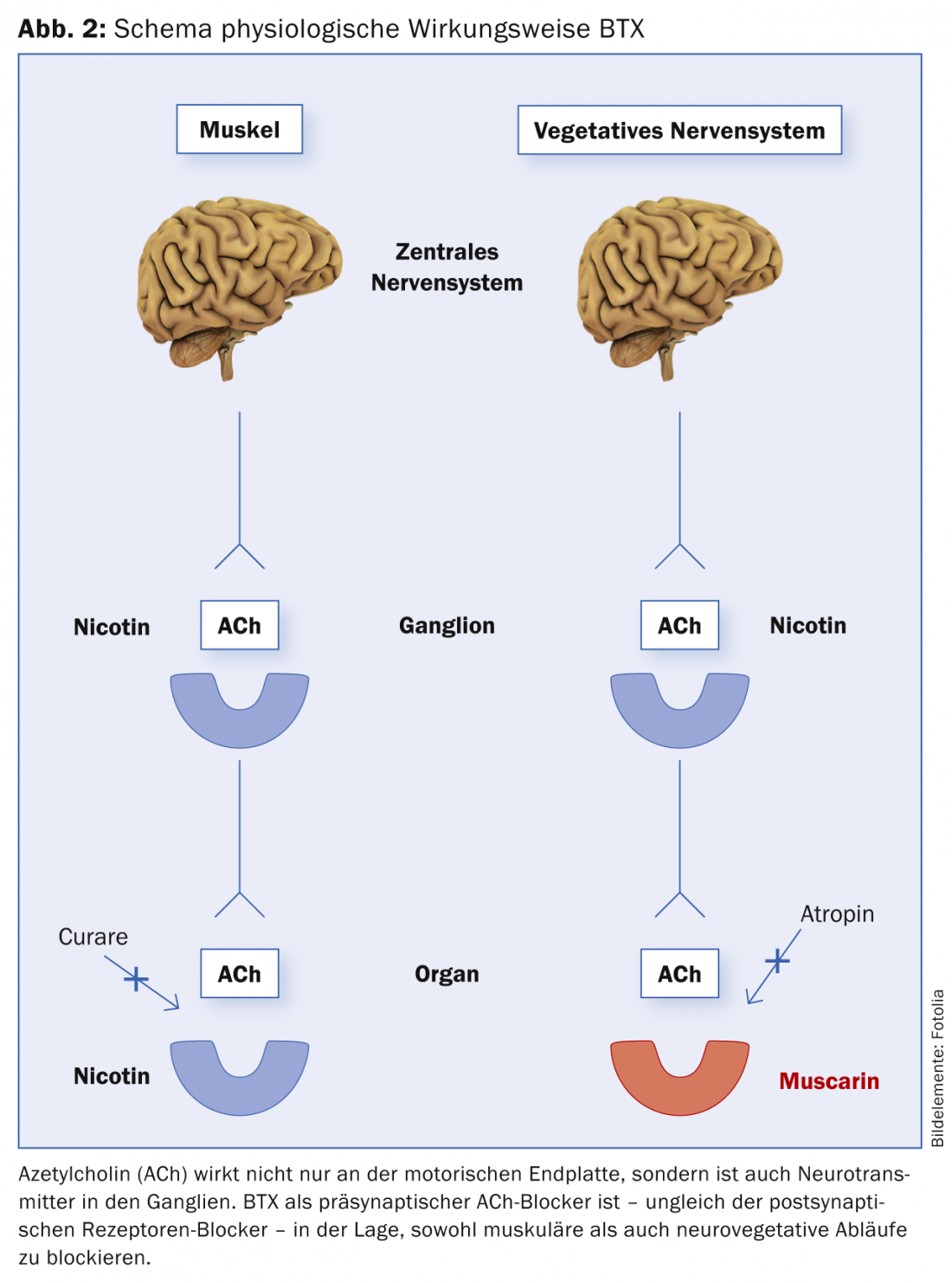
The toxin molecule consists of a light chain (L chain) with a molecular weight of 50 kD (kilo Dalton) and a heavy chain (H chain) with a molecular weight of 100 kD. The chains are connected by a disulfide bridge. The H-chain is responsible for the so-called internalization: the uptake of the toxin into the nerve terminal in the area of the presynaptic end plate. After endocytosis, the L-chain splits off from the H-chain. The L-chain cleaves the protein SNAP-25, a component of the so-called SNARE fusion complex (Soluble N-ethylmaleimide-sensitive-factor Attachment Receptor, SNARE) at various sites. As a result, fusion of the ACh-containing vesicle with the membrane is no longer possible, and thus no release of ACh from the cholinergic nerve terminal is possible [10,11]. Thus, the discharge of the transmitter substance ACh is prevented and a postsynaptic effect is absent. However, the therapeutic effect is of limited duration, since new nerves later sprout again and new synaptic connections are established.
The preparation of the botulinum solution for treatment
There are several ways to dissolve the dry substance BTX. Many authors choose a dilution of 1 ml to 100 U Ona- and Incobotulinum resp. 500 U Abobotulinum for the treatment of mimic wrinkles with the aim of achieving the most precise injection result of the toxin with the minimum diffusion into unwanted muscles. However, this highly concentrated low-volume dissolution scheme has the major disadvantage that fine gradations in dosage and thus some “modeling” with the BTX dose are difficult to achieve. If 100 U BTX are dissolved in 1 ml, then 0.1 ml corresponds to 10 U BTX. The dose of an injection point for the treatment of mimic wrinkles is usually between 2 U and 6 U. Thus, a high level of experience and a sure hand is required when injecting small doses. Therefore, dilution of BTX for the different indications as shown in Table 2 and 3 has been established.
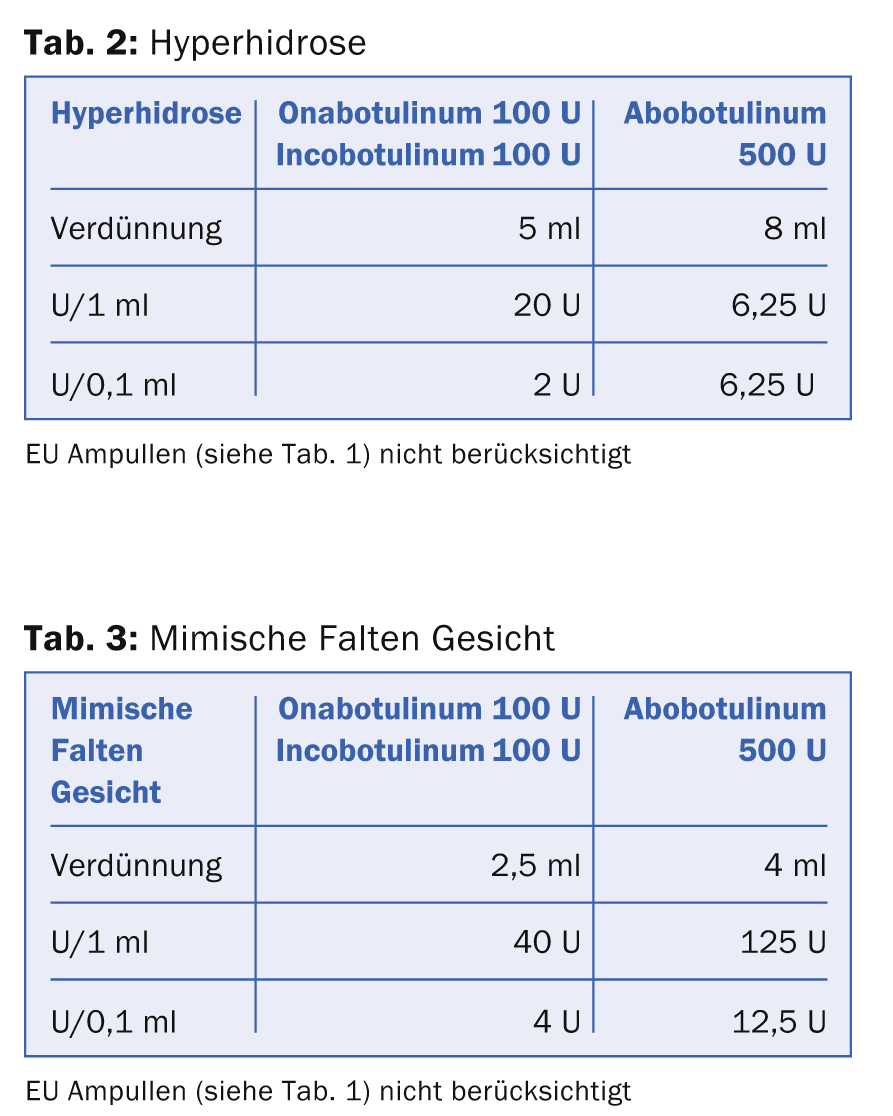
The tables show that the equivalent dose of Ona- resp. Incobotulinum and Abobotulinum is calculated as 1:3. The special thing about reconstituting Incobotulinum is that the company strongly recommends tilting the vial several times after triggering to achieve maximum effect of the drug solution.
What material do I need for BTX treatment?
The material required for treatment with BTX is relatively manageable. Correct and well-planned preparation leads to safety and increase in quality. Or, to put it the other way around, if the doctor has to gather this and that before the planned procedure or even interrupt the treatment because of a lack of preparation, there are per se dangers for incorrect treatments. It must be remembered that we are dealing with a highly potent drug. The instruction of the assistants is of utmost importance.
For a correct BTX treatment it needs:
- 1 original pack of BTX (Ona-, Inco or Abobotulinum) 100 U
- NaCl 0.9% 5 ml (hyperhidrosis) resp. 2.5 ml (esthetic indication) Ona- and Incobotulinum, 8 ml resp. 4 ml Abobotulinum (Cave: in the EU 50 U resp. 125 U Flacon available, thus the volume quantity is halved)
- 5 ml syringe with 18 G needle (pink) for reconstitution
- Insulin syringes (1 ml) without attached needle with scale in ml
- 32G needle attachable for the purpose of injection
- Disinfection both spray, e.g. Kodan® for the treatment of hyperhidrosis and liquid, e.g. Octenisept® for the treatment of the face
- Face headband
- Utensils for Minor’s sweat test for the treatment of axillary hyperhidrosis:
- Lugol’s solution (pharmacy)
- Potato starch (pharmacy)
- Double sieve for the finest possible application of potato starch (department store)
- Kajal pencil (soft!) for drawing in the injection points
- make-up removal emulsion and washing possibility after performed treatment (especially after axillary hyperhidrosis)
This list can of course be adapted to personal preferences. The used syringes, needles and empty vials must be disposed of separately! This is a point not to be omitted! BTX and residues thereof must not be disposed of with general waste.
To be continued…

Literature:
- Dickson EC: Botulism: A clinical and experimental study. Rockefeller Institute for Medical Research 1918; 1-117.
- Kerner JC: Poisoning from tainted sausages. Tübinger Blätter für Naturwissenschaften und Arzneykunde 1817; 3: 1-45.
- Kreyden OP, Geiges ML, Burg G: Botulinum toxin: from poison to drug. A historical review. Dermatologist 2000; 51: 733-737.
- Burgen A, Dickens F, Zatman LJ: The action of botulinum toxin on the neuromuscular junction. J Physiol 1949; 109: 10-24.
- Scott AB: Botulinum toxin injection of eye muscles to correct strabismus. Trans Am Ophthalmol Soc 1981; 79: 734-770.
- Carruthers JD, Carruthers JA: Treatment of glabellar frown lines with C. botulinum-A exotoxin. J Dermatol Surg Oncol 1992 Jan; 18(1): 17-21.
- Bushara KO, et al: Botulinum toxin–a possible new treatment for axillary hyperhidrosis. Clin Exp Dermatol 1996 Jul; 21(4): 276-278.
- Kreyden OP, Böni R, Burg G: Hyperhidrosis and Botulinum Toxin in Dermatology. Current Problems in Dermatology Karger 2002. ISBN 0070-2064.
- Barker WH: Botulism. The Cambridge World History of Human Disease. Kiple, KF and Graham 1993; 623-625.
- Kao I, Drachman DB, Price DL: Botulinum toxin: mechanism of presynaptic blockade. Science 1976; 193: 1256-1258.
- Philipp-Dormston WG: Botulinum toxin in dermatology. Der Hautarzt 2014; 65: 133-145.
DERMATOLOGIE PRAXIS 2015; 25(1): 29-34