Not all brain tumors are the same. The spectrum is broad. The goals of neurosurgery are to enable definitive histopathologic diagnosis by tissue sampling, symptom improvement by relieving the mass, and improved prognosis by reducing tumor burden.
Physicians in private practice, in terms of incidence and prevalence of brain tumors, are rarely confronted with this diagnosis. Therefore, some uncertainties exist regarding the approach to this inhomogeneous group of diseases. The spectrum of brain tumors ranges from small and benign and in principle curable tumors to fast and infiltrative growing variants with still poor prognosis. The purpose of this article is to provide a basic overview of the most common types of brain tumors. From this, basic features of diagnostic and therapeutic neurosurgical options will be presented.
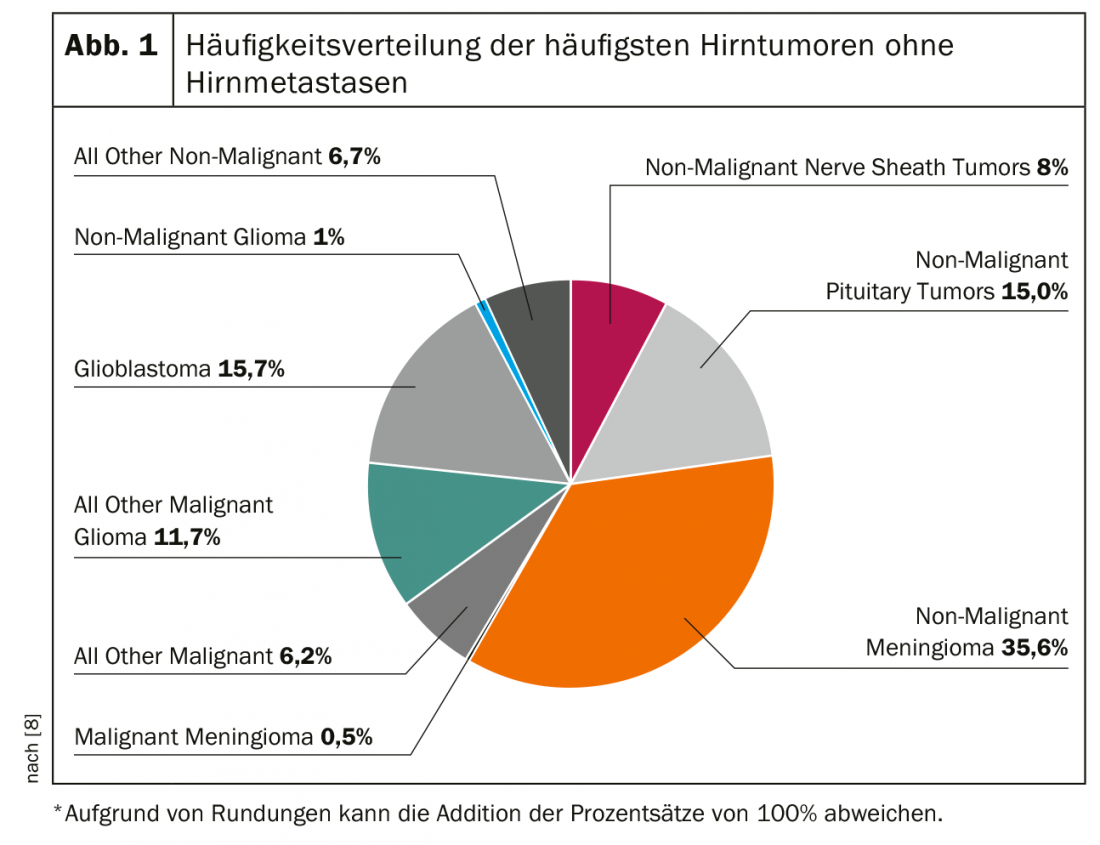
In practice, it is either incidental findings discovered in the course of imaging diagnostics of other suspected diagnoses or spatial masses imaged in the course of clarifying new, mostly neurological symptoms that lead to the suspected diagnosis of a brain tumor. Figure 1 graphically depicts the frequency distribution of various brain tumors. It is worth mentioning that brain metastases are ultimately the most common entity, but are not counted as “brain tumors” in most statistics.
In principle, a distinction must be made between tumors that are intrinsic to the brain, i.e. primary brain tumors, and tumors originating from other tissues. The latter may originate, for example, from the meninges (“meningiomas”) or may be metastatic metastases of other tumors. Therefore, in the case of suspected intracranial metastasis with previously unknown primary tumor, the search for a potential primarius must also be considered. Brain tumors, mostly belonging to the large group of astrocytic or oligodendroglial gliomas, are usually diffusely growing neoplasms without clear demarcation to the surrounding brain tissue. This also explains the therapeutic difficulties, since a curative approach with resection “in the healthy” is practically impossible. Non-brain tumors usually have a separating layer from the brain that can be identified by the neurosurgeon and are therefore more operable from a technical point of view.
Therapy management options
The basic therapeutic options for brain tumors are surgery, radiation treatment, and chemotherapy. Magnetic resonance imaging (MRI) is the primary imaging modality used, and specialized neuroradiology can increase diagnostic power noninvasively with newer developments such as MR perfusion or MR spectroscopy. For almost all intracranial tumors, internationally accepted treatment algorithms have evolved, usually involving multiple diagnostic and therapeutic disciplines. Therefore, the treatment of brain tumors should take place in specialized tumor centers that can offer all potentially necessary modalities and disciplines and, based on their case numbers, have the necessary expertise. The concept of a tumor center also includes that all patients are presented in a neuro-oncological tumor conference and that the diagnostic and therapeutic procedure is determined interdisciplinary. In addition, patients also benefit from other services, such as psycho-oncological care or social services, if desired and necessary.
Therapeutic goal: removal of the tumorous tissue
With few exceptions of tumors that are primarily treated by radiooncology and/or oncology (for example, lymphomas), the primary therapeutic goal is to remove as much of the tumorous tissue as possible (Fig. 2). Preservation of neurological functions, such as speech, is essential when planning potential surgery, as continued quality of life depends critically on this. Therefore, the assessment of operability of brain tumors must always be done in terms of neurological function preservation. In cases of functionally critically located brain tumors, resection is therefore occasionally omitted and only a biopsy is performed to establish the diagnosis. In the case of multiple intracranial tumor manifestations, e.g., in the case of suspected multiple brain metastases, often only bioptic diagnostic confirmation is sought as well.
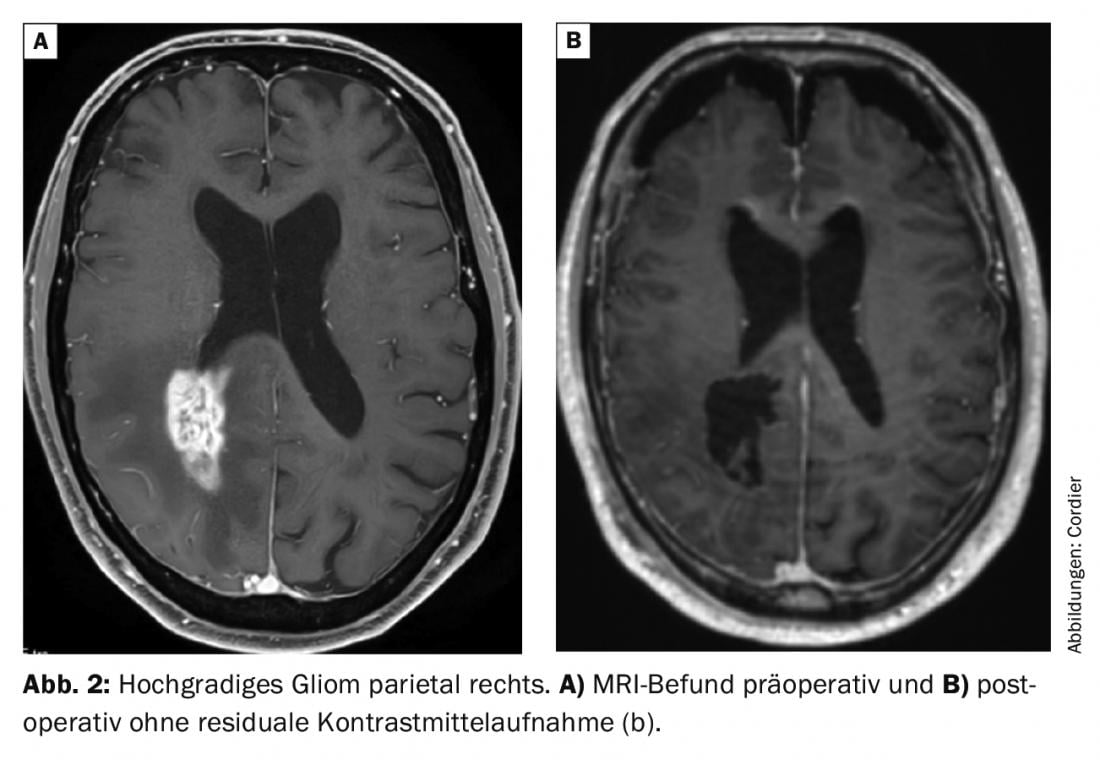
Surgery is at the forefront of the treatment algorithm for most brain tumors because it is (A) allows the definitive diagnosis to be made, (B) often improves symptoms by relieving the space-occupying effect on the brain; and (C) brings a relevant improvement in prognosis in many cases [1].
The definitive diagnosis by a specialized neuropathologist is of high importance, as it significantly influences further therapy planning and allows a better prognosis assessment than, for example, the sole analysis of different imaging modalities. In particular, molecular genetic analysis of the resected tissue allows for differentiated subtyping [2].
Prognosis improvement by resection
The space-occupying effect of a brain tumor arises on the one hand from actual substance proliferation within the closed cranial cavity, and on the other hand also from tumor-induced edema in the surrounding brain tissue. Resection of tumor tissue not only reduces the direct space-occupying lesion, it also removes the pathophysiologic basis of the disrupted blood-brain barrier and leads to regression of edema.
The aforementioned improvement in prognosis is closely related to the extent of resection that can be achieved. For non-brain tumors such as meningioma, complete tumor removal, including the site of origin at the dura mater, is often possible. This allows long-term recurrence rates of less than 10% [3]. The assessment of the long-term probability of recurrence of meningiomas is based on the extent of resection achieved (grading according to Simpson) and the WHO histopathological classification revised in 2016, as in rare cases meningiomas may also show increased mitotic rates [4]. Although metastases are not actually “brain” tumors, they can still break through the separating layer to the brain (pia mater) and grow invasively. Accordingly, a search must be made for any tumor cones infiltrating the brain. In cases of multiple metastases, careful consideration must be given to whether surgery can improve prognosis. In any case, diffuse brain-infiltrating gliomatous tumors are problematic, in which a complete surgical removal of all tumor cells is practically impossible. With regard to fast-growing high-grade gliomas, especially glioblastoma as the most aggressive variant, a required resection extent of at least 90% is assumed and recommended to achieve a relevant prognostic improvement [5]. This means that preoperatively the objective, the prospects of success and the risks of the operation must be discussed comprehensively with the patient. Slow-growing, low-grade gliomas represent a special form. Function-preserving partial resection is also often performed for these tumors due to their slow progression [6].
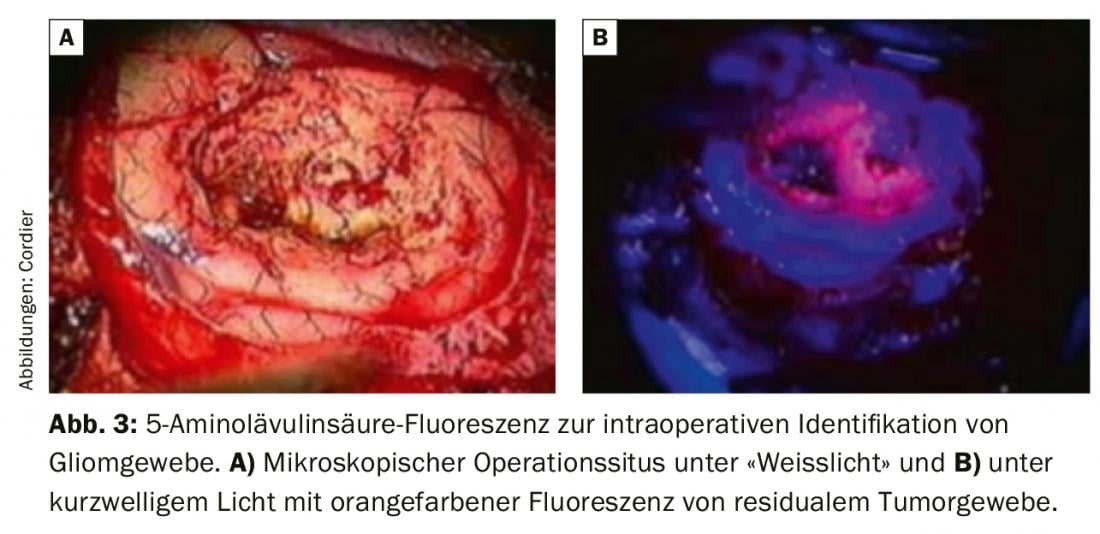
In recent years, several technical developments have led to a significant increase in the extent of resection, particularly in infiltrative tumors. The use of surgical microscopes has been standard for decades and, with excellent illumination, enables safe preparation that is appropriate for the sensitive tissue. Preoperative planning often includes a virtual three-dimensional reconstruction of the tumor and the surrounding anatomical structures to be spared, so that these can be localized more precisely intraoperatively using neuronavigation. When the presence of a fast-growing higher-grade glioma is suspected, the preoperative oral administration of 5-aminolevulinic acid has become established, which causes the tumor cells to fluoresce in contrast to their non-tumor neighboring cells when illuminated with short-wave light (Fig. 3). This often allows macroscopically “normal” appearing tissue to be identified as tumor tissue [7]. In addition to the functional importance of different cortical areas, the importance of subcortical fiber tracts as a connection between different cortical centers has been increasingly demonstrated in recent years. The MRI-based diffusion tensor imaging technique allows preoperative three-dimensional visualization of the large subcortical fiber tracts. This is incorporated into the preoperative planning of tumor resection and supports an increase in the extent of resection while preserving function. Intraoperative electrophysiological studies are performed with the same goal. This usually involves monitoring different cranial nerves (e.g. facial nerve) or identifying primary motor or sensory areas. The best possible identification and sparing of functionally critical brain structures is achieved by tumor resection while the patient is awake under neuropsychological monitoring. The main advantage of this technique is that not only “primary” brain functions can be monitored intraoperatively, but also higher integrative brain functions such as complex linguistic, mathematical or mnestic abilities can be studied. The latter technique is used primarily for slow-growing brain tumors (low-grade gliomas), since the goal of preserving function also justifies limiting the extent of resection. Due to the cerebral reorganization of brain functions in slow-growing gliomas, the so-called plasticity of the brain, it is often possible to operate again without neurological deficits in case of tumor progression.
Take-Home Messages
- The spectrum of differential diagnoses in brain tumors is broad; a basic classification into brain and non-brain tumors is helpful.
- Patients with a suspected diagnosis of a brain tumor should be referred to a specialized interdisciplinary brain tumor center for further diagnosis and therapy.
- The objectives of neurosurgery are to enable definitive histopathologic diagnosis by tissue sampling, symptom improvement by relieving the mass, and improved prognosis by reducing tumor burden.
Literature:
- Hameed NUF, et al: Transcortical insular glioma resection: clinical outcome and predictors. 2018: 1.
- Rogers TW, et al: The 2016 revision of the WHO Classification of Central Nervous System Tumours: retrospective application to a cohort of diffuse gliomas. Journal of Neuro-Oncology, 2018. 137(1): 181-189.
- Lam Shin Cheung V, et al: Meningioma recurrence rates following treatment: a systematic analysis. Journal of Neuro-Oncology, 2018. 136(2): 351-361.
- Louis DN, et al: The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathologica, 2016. 131(6): 803-820.
- Sanai N, Berger MS: Surgical oncology for gliomas: the state of the art. Nature Reviews Clinical Oncology, 2017. 15: 112.
- Duffau H, Mandonnet E: The “onco-functional balance” in surgery for diffuse low-grade glioma: integrating the extent of resection with quality of life. Acta Neurochirurgica, 2013. 155(6): 951-957.
- Stummer W, van den Bent MJ, Westphal M: Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochirurgica, 2011. 153(6): 1211-1218.
- Ostrom QT, et al: CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007-2011. Neuro Oncol, 2014. 16 Suppl 4: iv1-63.
InFo ONCOLOGY & HEMATOLOGY 2019; 7(2-3): 20-22.