Approximately 50% of perioperative causes of death in noncardiac surgery can be attributed to cardiovascular causes. Appropriate preoperative cardiovascular risk assessment can reduce the risk of complications. To this end, the authors of the ESC Guideline 2022 have developed specific recommendations based on a comprehensive review of the current literature.
As the population ages, so does the number of conditions requiring surgical intervention. Accordingly, you are very likely to encounter more and more patients requiring preoperative evaluation in your own waiting room. The question then arises as to which examinations are indicated and necessary preoperatively. Which of our patients may be operated on without hesitation, and which may still require therapy before the actual operation (surgery)? For this very issue, the European Society of Cardiology (ESC) “Guideline on cardiovascular assessment and management before non-cardiac interventions” [1], published in 2022, provides guidance for action. The goal of this guideline is to reduce perioperative mortality and morbidity using a standardized and evidence-based systematic approach. This is because if we look at the exact causes of death of the four million patients who die perioperatively each year, we find that about 50% are due to cardiovascular causes [2,3].
Specifically, recommendations were made for patients with new heart murmurs, shortness of breath, edema, or angina. Furthermore, special focus is given to the assessment of frailty/frailty of patients and the collection of the biomarkers brain natriuretic peptide or N-terminal pro brain natriuretic peptide (hereafter both abbreviated as BNP) and troponin. Recommendations are also made for the management of anticoagulant medication, bleeding, and perioperative cardiovascular complications. For the first time, the guideline addresses patients with underlying malignancies and with covid-19 infection. The following is a summary of the most important aspects for your daily clinical routine.
The first step: The preoperative risk assessment
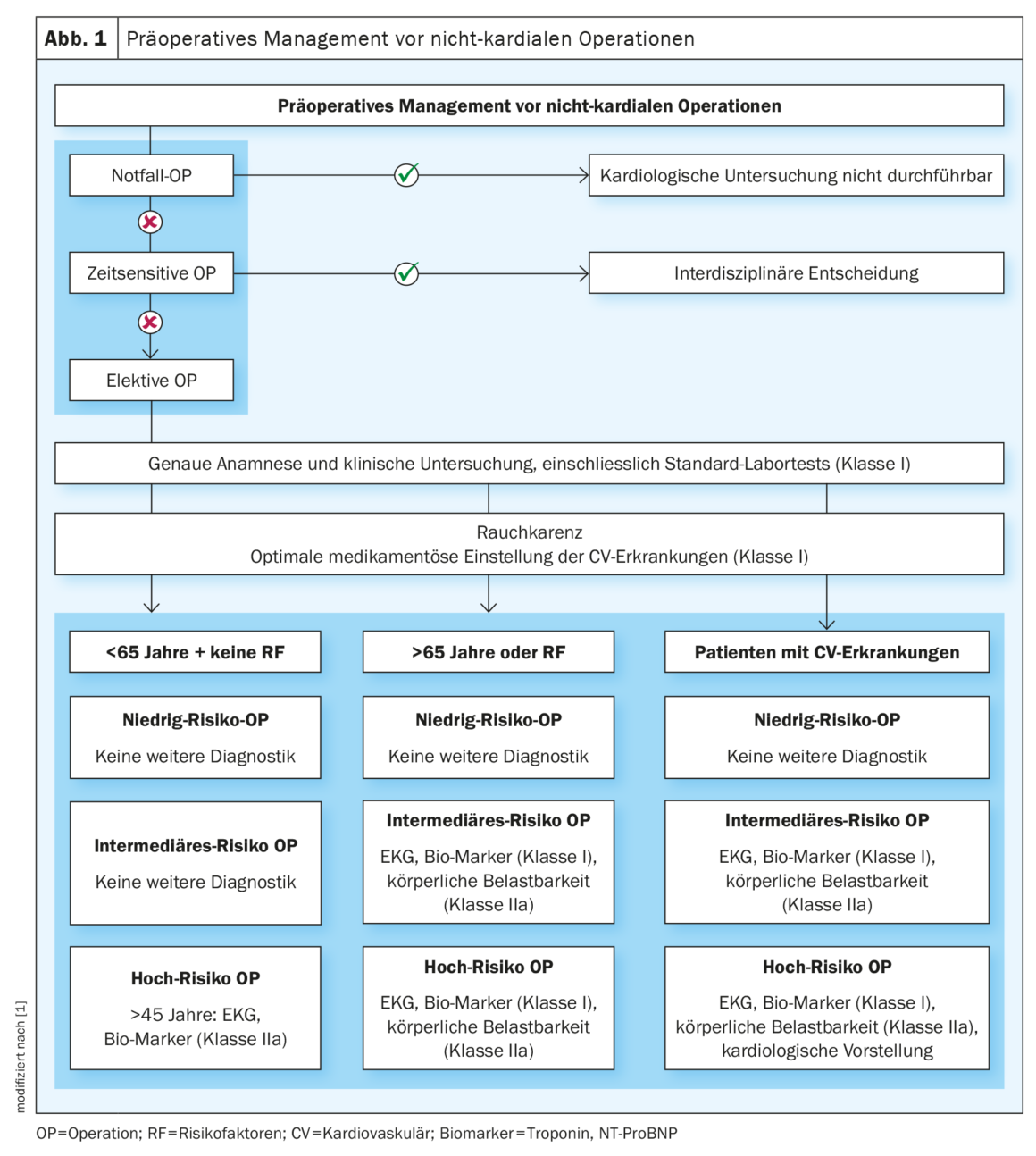
As shown in Figure 1 , the algorithm first recommends ranking the urgency of the operation. A distinction is made between emergency, urgent, prompt and elective. For emergency and urgent surgeries, the opportunity for risk stratification is limited and requires bedside interdisciplinary assessment when the situation allows. However, the majority of clinical circumstances allow the systematic approach recommended in the guideline. The basis of the further diagnostic and treatment pathway is the medical history, clinical physical examination, and a standard laboratory, which should include at least a blood count and renal function.
In all patients, optimization of cardiovascular risk factors is a high priority before planned surgery. Preoperative nicotine abstinence of at least four weeks should be advised. This showed a significantly improved surgical outcome, especially with regard to wound infections [4]. Drug therapy for arterial hypertension, dyslipoproteinemia, and diabetes mellitus is also useful, but weight reduction immediately before surgery is not.
Subsequently, based on the patient-associated risk and the risk of the planned surgery, further preoperative diagnostics can be adjusted and supplemented, for example, with 12-lead electrocardiography (ECG) and cardiac biomarkers (troponin, BNP). Data show a prognostic association of biomarkers with postoperative cardiovascular complications [5]. BNP can help diagnose undetected heart failure [6] and troponin can diagnose cardiac ischemia or postoperative infarction [7].
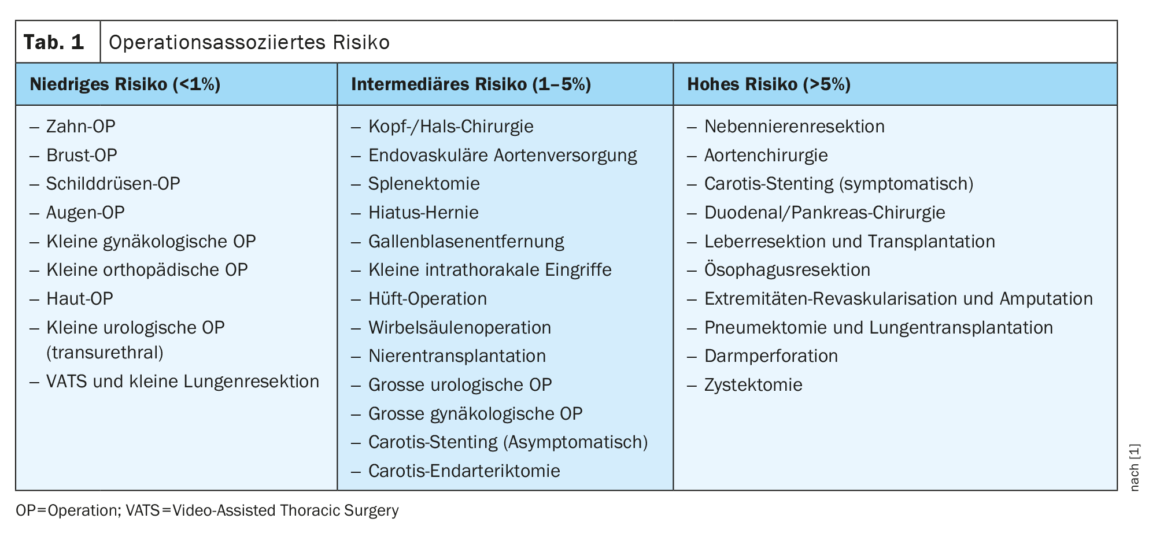
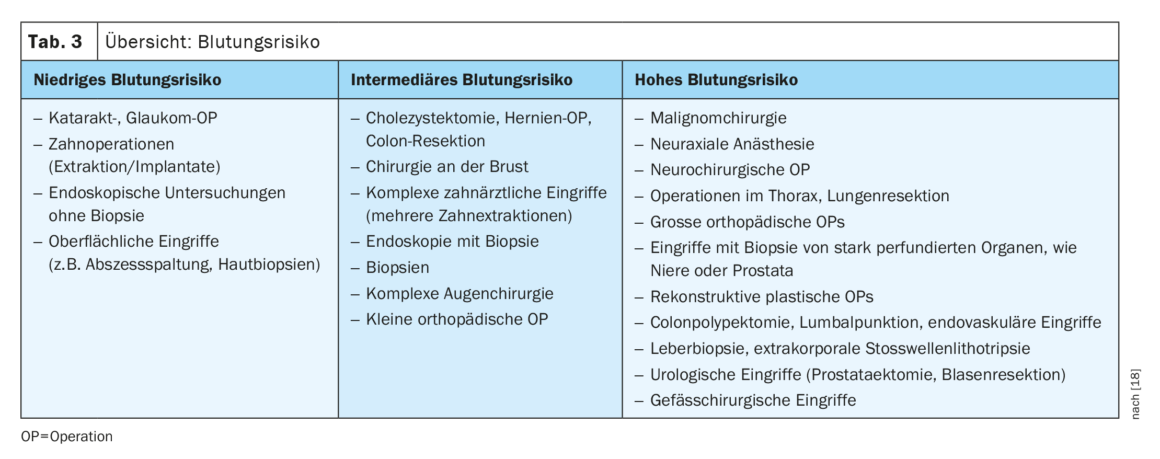
The guideline defines three patient categories and three surgery risk categories: Surgery-associated risk is categorized as low-risk, intermediate-risk, and high-risk. Multiple examples are provided in the guideline for each category, as also found in Table 1. Three factors are considered in patient-associated risk: Age, cardiovascular risk factors, and previous cardiovascular disease [8,9]. It is divided as follows, with appropriate recommendations for further diagnostics depending on the surgery-associated risk:
- In patients between the ages of 45 and 65 years without risk factors or preexisting conditions, evaluation by ECG and biomarkers should be performed only before surgery with high operative risk (class IIa) [8]. Exception: if there is a family history of genetic cardiomyopathy, an ECG and transthoracic echocardiography (TTE) should be performed in young, asymptomatic patients.
- In patients aged 65 years or older or with existing risk factors (hypertension, smoking history, hyperlipoproteinemia, family history, diabetes mellitus), ECG (class I) and biomarker (class I) collection are already recommended for planned intermediate-risk surgery. In addition, exercise capacity (class IIa) should be evaluated to detect possible cardiovascular disease. This can be determined, for example, by the Duke Activity Status Index [10] or the ability to climb two flights of stairs. An exercise ECG under ergometric stress shows a low specificity and should only be used as an alternative if the physical capacity cannot be validly determined from anamnesis [9].
- In patients with known prior cardiovascular disease, all recommendations as listed in 2 are recommended. In addition, in high-risk surgery, the decision on the further procedure should be made in interdisciplinary consultation with the treating cardiologist, taking into account all the information and findings.
Advanced diagnostics
Findings such as a heart murmur, shortness of breath, angina pectoris, or edema may be initial indications of relevant, unknown cardiovascular disease [9]. If those show up on clinical examination, the following is recommended:
New heart murmur
- With symptoms: TTE (Class I)
- Without symptoms: TTE before intermediate- or high-risk surgery (class IIa).
Angina pectoris
- Elective surgery: advanced cardiological diagnostics (class I)
- Urgent OP: ECG, biomarkers, and interdisciplinary discussion (Class I).
Shortness of breath and/or peripheral edema
- ECG and biomarkers (Class I)
- TTE in the presence of elevated biomarkers (Class I).
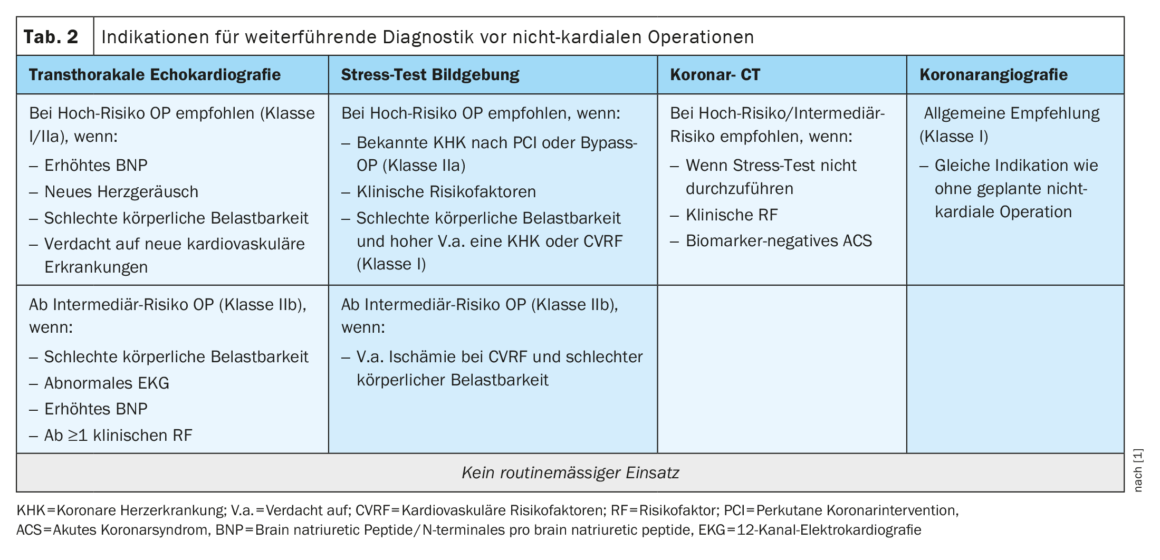
Impaired left ventricular (LV) function, valvular disease, and cardiomyopathies are the three entities with the greatest perioperative cardiac risk potential, which are easily detected by transthoracic echocardiography [12]. Impaired LV function (systolic as well as diastolic) also plays a crucial role regarding postoperative cardiac complications [13]. However, TTE is not assigned to a category in the central diagnostic pathway as a general recommendation. However, the guideline specifies specific indications for their implementation (Table 2) .
In patients with poor exercise capacity, clinical risk factors, and abnormal echocardiography, stress imaging may be performed for further diagnosis [14]. A perfusion deficit on stress imaging shows an association with an increased rate of postoperative cardiac complications [15]. If stress imaging is not possible, coronary computed tomography (CT) can be performed to rule out relevant CHD with low pretest probability and expected good image quality [16]. Coronary angiography should be indicated along the guideline for “Myocardial Revascularization” regardless of the planned elective surgery [11].
The Perioperative Myocardial Infarction/Injury (PMI).
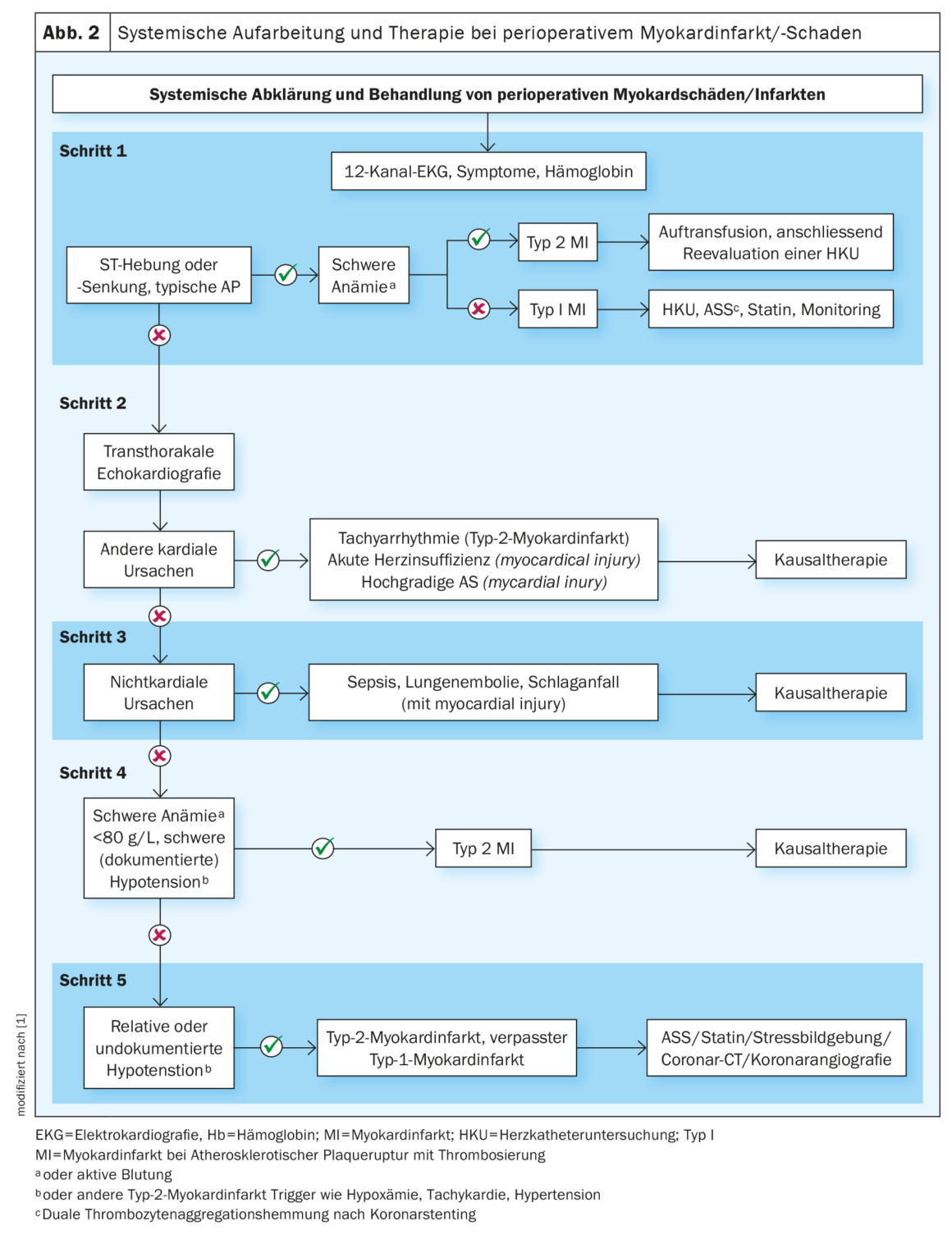
Troponin is commonly established, favorable, excludes cardiac ischemia at normal values, and aids in the diagnosis of perioperative myocardial infarction/damage (PMI) [7]. The latter is one of the most common cardiovascular complications seen. In 90% of cases, it remains initially asymptomatic due to anesthesia and postoperative analgesia or is masked by other symptoms, such as wound pain, making it difficult to diagnose [17]. To counteract this, troponin sampling is recommended 24 h and 48 h (class I) after surgery in addition to preoperative sampling in the appropriate patient population. If elevated values are found, further clarification is indicated to determine the cause. Significantly elevated troponin is defined as an increase above the upper limit of the assay used in the particular laboratory. Indeed, PMI may be due to a cardiac cause, such as type I or type II myocardial infarction, acute decompensation, or tachyarrhythmia, as well as a noncardiac cause, such as severe sepsis or pulmonary artery embolism. For this purpose, the algorithm given in this guideline should be used and, depending on the cause, the appropriate therapy should be initiated (Fig. 2). In cases of highly probable myocardial infarction due to atherothrombosis, coronary angiography should be performed immediately after exclusion of severe anemia. Routine determination of biomarkers is not recommended.
Recommendations for blood thinning and antiplatelet therapy
The new guideline formulates recommendations for the management of plasmatic anticoagulants and antiplatelet agents for perioperative risk reduction. The first step is to evaluate the perioperative bleeding risk (Table 3). Subsequently, the patient’s thrombotic risk must be assessed.
Platelet aggregation inhibition
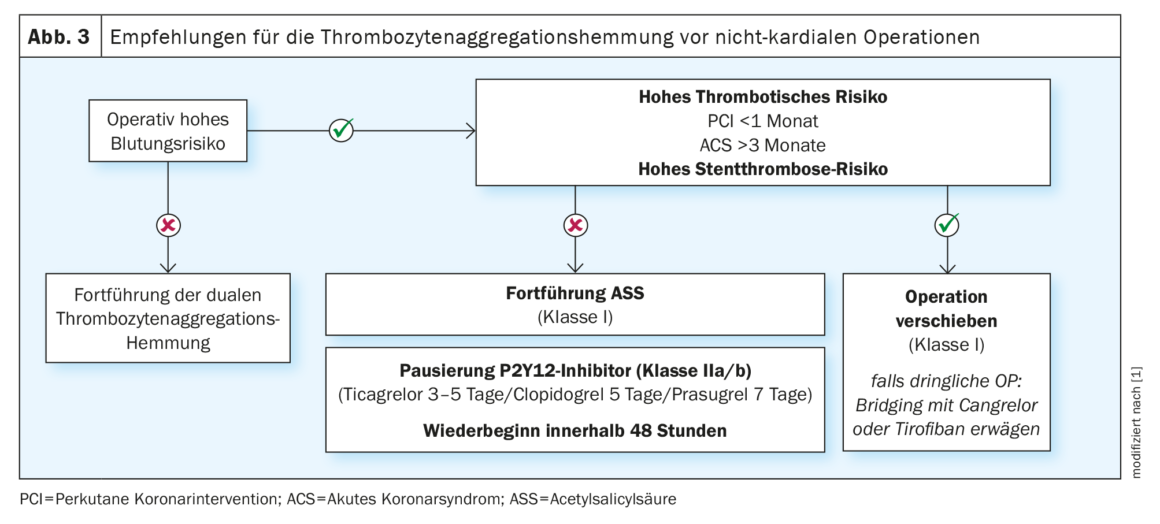
When acetylsalicylic acid (ASA) is taken as a primary prophylactic agent, the risk of cardiovascular events is low and use can be paused perioperatively [19]. For secondary prophylactic use, on the other hand, pausing is not recommended (Class III) [20]. Patients on dual antiplatelet therapy after percutaneous coronary intervention (PCI) have a significantly increased risk of relevant cardiovascular complications of 2-8% [21]. The highest risk here is in the first month after PCI, especially if the initial PCI was in STEMI, dual antiplatelet therapy was paused, or the coronary lesion was complex [22]. Therefore, elective noncardiac surgery should be postponed until after cessation of dual antiplatelet therapy (six months after elective PCI and 12 months after acute coronary syndrome (ACS) or high-risk PCI [Klasse I]) [23]. For urgent surgical indications, dual antiplatelet therapy should have been taken for at least three months (class IIb) in high-risk patients after PCI in the setting of ACS and for at least one month (class IIa) after elective PCI. Subsequently, only the P2Y12 inhibitor should be paused for three to seven days preoperatively [24] and restarted with loading dosing as soon as possible after risk evaluation (Table 3). In high-risk constellations after PCI, noncardiac surgery should be performed at a center with 24-hour cardiac catheterization laboratory capacity.
Oral anticoagulation
With combination therapy of antiplatelet therapy and oral anticoagulation, elective surgery should be postponed until the end of antiplatelet therapy (six months after elective PCI and 12 months after ACS). When taking a vitamin K antagonist, if the risk of bleeding is higher and the risk of thrombosis is also high (for example, in patients with mechanical valve replacement), the drug should be paused and bridging therapy with heparin should be initiated [25]. In patients on oral anticoagulation for atrial fibrillation, bridging showed a negative effect, so it should be performed here only when the thrombotic risk is high and weighed against the bleeding risk [26]. Resumption of OAK can be risk-adjusted starting twelve hours postoperatively. Non-vitamin K
Antagonists should also be paused before noncardiac surgery. Bridging is indicated only in exceptional cases of high thrombotic risk. In case of impaired renal function, the intake should be discontinued earlier (GFR <50 mL/min at least 48-72 hours) [27].
Special diseases
Patients’ preexisting conditions should also be considered preoperatively, as they also favor perioperative risk: Coronary artery disease increases the perioperative risk. ACS should always be managed according to guideline and elective surgery should be postponed for it [28]. In chronic coronary syndrome, there is still no safe recommendation in the absence of data [29]. Heart failure also negatively affects postoperative mortality after noncardiac surgery [30]. Guideline-based drug therapy should be initiated and continued [31]. An existing CRT device should not be deactivated except for the defibrillation function.
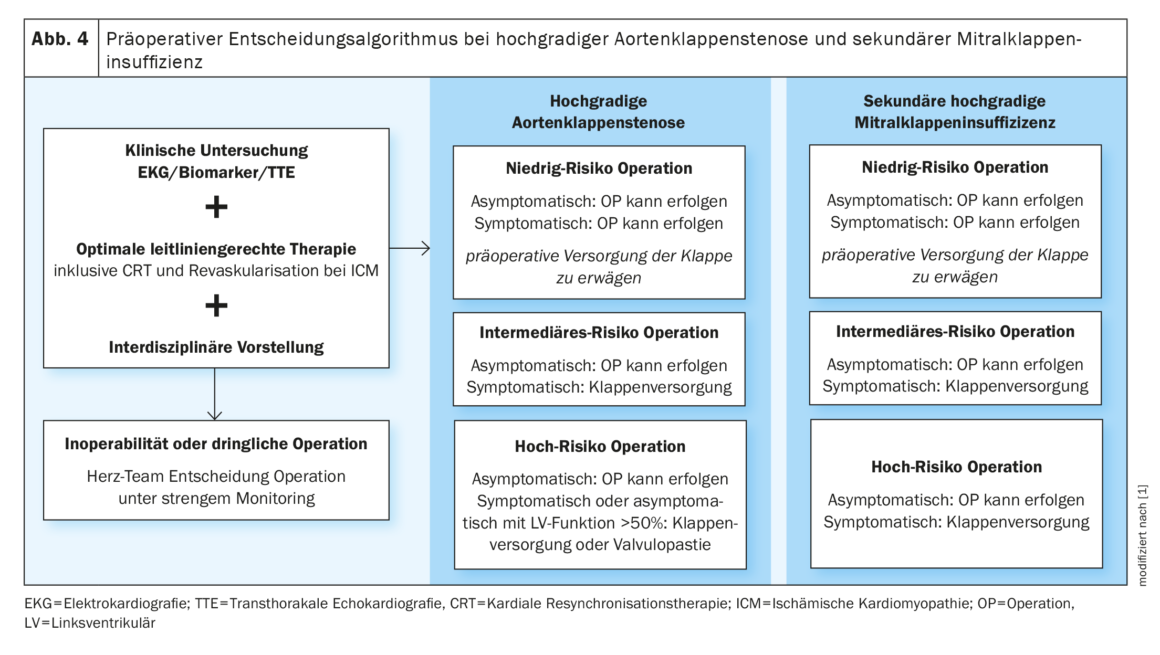
Valvular heart disease, depending on the severity of the vitium, increases the perioperative risk and therefore poses a major challenge preoperatively [32]. Therefore, TTE should be performed in all patients with known valvular disease before noncardiac intermediate- to high-risk surgery. The following recommended action is stated in the guideline:
Symptomatic high-grade aortic stenosis (AS) has been shown to adversely affect postoperative complications and 30-day survival [33]. Because of this, elective surgery should be postponed until after valve replacement. For urgent noncardiac surgery, balloon valvuloplasty may serve as a bridge procedure. Patients with asymptomatic, high-grade AS with normal LV function may be cleared for low- and intermediate-risk surgery.
In high-grade, symptomatic aortic valve regurgitation (AI) as well as high-grade, asymptomatic AI (with an LVESD >50 mm or left ventricular function (LVF) of >50%, guideline-guided valve repair should be performed before elective intermediate or high-risk surgery [34].
Balloon valvuloplasty is recommended before high-risk surgery in symptomatic, moderate-to-high-grade mitral valve stenosis and when systolic pulmonary arterial pressure is >50 mmHg.
In cases of high-grade mitral regurgitation (MI), the first step is to clarify LVF and etiology. In the presence of concomitant ischemic cardiomyopathy and secondary MI, valve intervention should again be performed before elective surgery in the intermediate or high-risk setting [35,36].
Active pericarditis should first be treated according to guidelines before noncardiac surgery [37].
Patients with pulmonary disease have pulmonary complications, especially postoperatively. In patients with chronic obstructive pulmonary disease, the preoperative period should be used to initiate antiobstructive therapy to improve pulmonary function.
If obstructive sleep apnea is present, the indication for continuous positive airway pressure therapy should be evaluated to reduce cardiovascular risk [38].
In patients with arterial hypertension with a blood pressure profile above 180 mmHg systolic, as well as above 110 mmHg diastolic, elective surgery should be postponed until improved control is achieved [38,39].
Patients with cerebral arterial occlusive disease combined with neurologic symptoms within the past six months should present preoperatively to neurology. In patients with symptomatic carotid stenosis (>70%) and transient ischemic attack or stroke in the last three months, it should be treated first [40].
If you have a patient with malignant disease to evaluate preoperatively, questions should include cardiotoxic chemotherapy and/or thoracic radiation, as these patients may present with CHD or vitiation, often at a young age [41].
With current or recent SARS-Cov2 infection, a higher number of thromboemboli can be observed, as well as higher mortality during noncardiac surgery. This risk persists for up to seven weeks after diagnosis and is particularly increased when patients are still symptomatic [42]. Furthermore, the resulting cardiac stress and associated myocardial damage leads to an increased perioperative risk of cardiac events [43]. Accordingly, elective surgery should be performed only after complete recovery. There are no recommendations yet for vaccinated patients in the absence of data.
Finally, it should be noted that for many recommendations in this guideline, only a level of evidence C is available and further studies are needed.
Take-Home Messages
- Approximately 50% of perioperative causes of death in noncardiac surgery can be attributed to cardiovascular causes. Through
appropriate preoperative cardiovascular risk assessment, the risk of complications can be reduced. - The authors of the 2022 ESC guideline developed 147 specific recommendations based on a comprehensive review of the current literature.
- A particular focus is on the collection of the biomarkers troponin and NT-ProBNP, as they show a prognostic association with postoperative cardiovascular complications.
- In the case of perioperative myocardial damage with troponin dynamics, the cause must be clarified and thus causal therapy made possible
- Individualized perioperative management of anticoagulation therapy can prevent thrombotic events and avoid bleeding complications.
- The guideline has also developed concrete recommendations on specific clinical pictures.
Literature:
- Halvorsen S, Mehilli J, Cassese S, et al.: ESC Scientific Document Group. 2022 ESC Guidelines on cardiovascular as-sessment and management of patients undergoing non-cardiac surgery. Eur Heart J 2022 Oct 14;43(39): 3826–3924.
- Weiser TG, et al.: An estimation of the global volume of surgery: a modelling strategy based on available data. Lancet 2008 Jul 12; 372(9633): 139–144.
- Smilowitz, et al.: Trends in cardiovascular risk factor and disease prevalence in patients undergoing non-cardiac surgery. Heart 2018 Jul; 104(14): 1180–1186.
- Gourgiotis, et al.: The effects of tobacco smoking on the incidence and risk of intraoperative and postopera-tive complications in adults. Surgeon 2011 Aug; 9(4): 225–232.
- Weber, et al.: Incremental value of high-sensitive troponin T in addition to the revised cardiac index for peri-operative risk stratification in non-cardiac surgery. Eur Heart J 2013 Mar; 34(11): 853–862.
- Mueller, et al.: Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentration, Eur J Heart Fail 2019;21: 715–731.
- Walter, et al.: Using High-Sensitivity Cardiac Troponin for the Exclusion of Inducible Myocardial Ischemia in Symptomatic Patients: A Cohort Study. Ann Intern Med 2020 Feb 4; 172(3): 175–185.
- Botto, et al.: Myocardial injury after noncardiac surgery: a large, international, prospective cohort study es-tablishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology 2014 Mar; 120(3): 564–578.
- Visseren, et al.: 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021 Sep 7; 42(34): 3227–3337.
- Wijeysundera, et al.: Assessment of functional capacity before major non-cardiac surgery: an international, prospective cohort study. Lancet 2018 Jun 30; 391(10140): 2631–2640.
- Knuuti, et al.: 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J 2020 Jan 14;41(3): 407–477.
- Steeds, et al.: EACVI appropriateness criteria for the use of transthoracic echocardiography in adults: a re-port of literature and current practice review. Eur Heart J Cardiovasc Imaging 2017; 18: 1191–1204.
- Halm, et al.: Echocardiography for assessing cardiac risk in patients having noncardiac surgery. Study of Perioperative Ischemia Research Group. Ann Intern Med 1996; 125: 433–441.
- Pellikka, et al.: Guidelines for performance, interpretation, and application of stress echocardiography in is-chemic heart disease: from the American Society of Echocardiography. J Am Soc Echocardiogr 2020; 33: 1–41.e8.
- Ballal, et al.: Prognosis of patients with vascular disease after clinical evaluation and dobutamine stress echocardiography; Am Heart J 1999;137: 469–475.
- Sheth T, Chan M, Butler C, et al.: Prognostic capabilities of coronary computed tomographic angiography before on-cardiac surgery: pro-spective cohort study. BMJ 2015; 350: h1907.
- Devereaux, et al.: Cardiac complications in patients undergoing major noncardiac surgery. N Engl J Med 2015; 373: 2258–2269.
- Steffel J, Collins R, Antz M, et al.: Guide on the use of non-vitamin k an-tagonist oral anticoagulants in patients with atrial fibrillation 2021 European Heart Rhythm Association Practical Europace 2021; 23: 1612–1676.
- Zheng, et al.: Association of aspirin use for primary prevention with cardiovascular events and bleeding events: a systematic review and meta-analysis. JAMA 2019; 321: 277–287.
- Graham, et al.: Aspirin in patients with previous percutaneous coronary intervention undergoing noncardiac surgery; Ann Intern Med 2018; 168: 237–244.
- Holcomb CN, Graham LA, Richman JS, et al: The incremental risk of coronary stents on postoperative adverse events: a matched cohort study. Ann Surg 2016; 263: 924-930.
- Saia, et al.: Risk of adverse cardiac and bleeding events following cardiac and noncardiac surgery in patients with coronary stent: how important is the interplay between stent type and time from stenting to surgery?; Circ Cardiovasc Qual Outcomes 2016; 9: 39–47.
- Knuuti, et al.: 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes, Eur Heart J 2020; 41: 407–477.
- Collet, et al.: 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021; 42: 1289–1367.
- Kuo, et al.: Thromboembolic and bleeding risk of periprocedural bridging anticoagulation: a systematic re-view and meta-analysis; Clin Cardiol 2020; 43: 441–449.
- Douketis JD, Spyropoulos AC, Kaatz S, et al.: Perioperative bridging antico-agulation in patients with atrial fibrillation. N Engl J Med 2015; 373: 823–833.
- Godier A, Dincq AS, Martin AC, et al.: Predictors of pre-procedural concentra-tions of direct oral anticoagulants: a prospective multicentre study. Eur Heart J 2017; 38: 2431–2439.
- Ibanez, et al.: 2017 ESC Guidelines for the management of acute myocardial infarction in patients present-ing with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177.
- Illuminati, et al.: Systematic preoperative coronary angiography and stenting improves postoperative results of carotid endarterectomy in patients with asymptomatic coronary artery disease: a randomised controlled trial.; Eur J Vasc Endovasc Surg 2010; 39: 139–145.
- Hammill BG, Curtis LH, Bennett-Guerrero E, et al.: Im-pact of heart failure on patients undergoing major noncardiac surgery, Anesthesiology 2008; 108: 559–567.
- McDonagh, et al.: 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure; Eur Heart J 2021; 42: 3599–3726.
- Agarwal, et al.: Impact of aortic stenosis on postoperative outcomes after noncardiac surgeries.; Circ Cardio-vasc Qual Outcomes 2013; 6: 193–200.
- Taniguchi, et al.: Elective non-cardiac surgery in patients with severe aortic stenosis – observations from the CURRENT AS Registry; Circ J 2020; 84: 1173–1182.
- Vahanian, et al.: 2021 ESC/EACTS Guidelines for the management of valvular heart disease; Eur Heart J 2022; 43: 561–632.
- Stone, et al.: Transcatheter mitral-valve repair in patients with heart failure; N Engl J Med 2018; 379: 2307–2318.
- Bauersachs J, et al.: 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Eur Heart J 2022;43: 561–632.
- Adler, et al.: 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC). Endorsed by: the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2015; 36: 2921–2964.
- Chau, et al.: Obesity hypoventilation syndrome: a review of epidemiology, pathophysiology, and perioperative considerations. Anesthesiology 2012; 117: 188–205.
- Im, et al.: Association between use of preoperative antihypertensive medication and 90-day mortality after noncardiac surgery: a retrospective cohort study; Am J Hypertens 2020; 33: 534–542.
- Aboyans, et al.: 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO). The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018;39: 763–816.
- 2022 ESC Guidelines on cardio-oncology ESC. Eur Heart J 2022;
- SARS-CoV-2 infection and venous thromboembolism after surgery: an international prospective cohort study. COVIDSurg Collaborative, GlobalSurg Collaborative. Anaesthesia 2022;77: 28–39.
- Guzik, et al.: COVID-19 and the cardiovascular system: implications for risk assessment, diagnosis, and treatment options. Cardiovasc Res 2020; 116: 1666–1687.
CARDIOVASC 2023; 22(2): 10–17