A KIT mutation analysis from the bone marrow is recommended in the case of highly elevated tryptase levels. In particular, adults often have a point mutation in codon 816 of the KIT gene. While symptom-oriented therapies target mast cell mediators, cytoreductive treatments can also be used for aggressive forms of systemic mastocytosis. The therapeutic target here is the reduction of neoplastic mast cell burden.
Mastocytosis refers to a heterogeneous group of diseases characterized by clonal proliferation of neoplastic mast cells in various tissues, particularly the skin and bone marrow. Less commonly, other organs such as the gastrointestinal tract, liver, spleen, lungs, and lymph nodes may be affected [1,2]. According to the World Health Organization classification updated in 2016, a distinction is made between cutaneous mastocytosis (CM), systemic mastocytosis (SM), and MC sarcoma (MCS) [3,17]. Prof. Dr. med. Karin Hartmann, Stv. Chief of Dermatology, Head of the Allergological Polyclinic, University Hospital Basel, gave an up-to-date overview of the diagnostic work-up and therapeutic options for systemic mastocytosis [3,17]. A variety of different stimuli (allergens, food components, infections, drugs, physical stimuli, insect venom) can trigger an uncontrolled release of mast cell mediators. These mediators (in addition to histamine and tryptase, heparin, leukotrienes, prostaglandins, and various cytokines) lead to heterogeneous and nonspecific symptoms. Systemic mastocytosis should be considered in patients with anaphylaxis, syncope, flushing, pruritus or brownish skin lesions and urticaria, as well as unexplained gastrointestinal symptoms and headache [4]. In most cases, the symptoms are rather mild and can be well controlled. However, mast cell activation syndromes (MCAS) with severe life-threatening conditions, such as anaphylactic shock, occasionally develop. In “advanced systemic mastocytosis” (AdvSM), there is often direct impairment of organ function due to mast cell infiltration itself and, in some cases, associated inflammation [3,5,6].
Screening: is there an increase in tryptase?
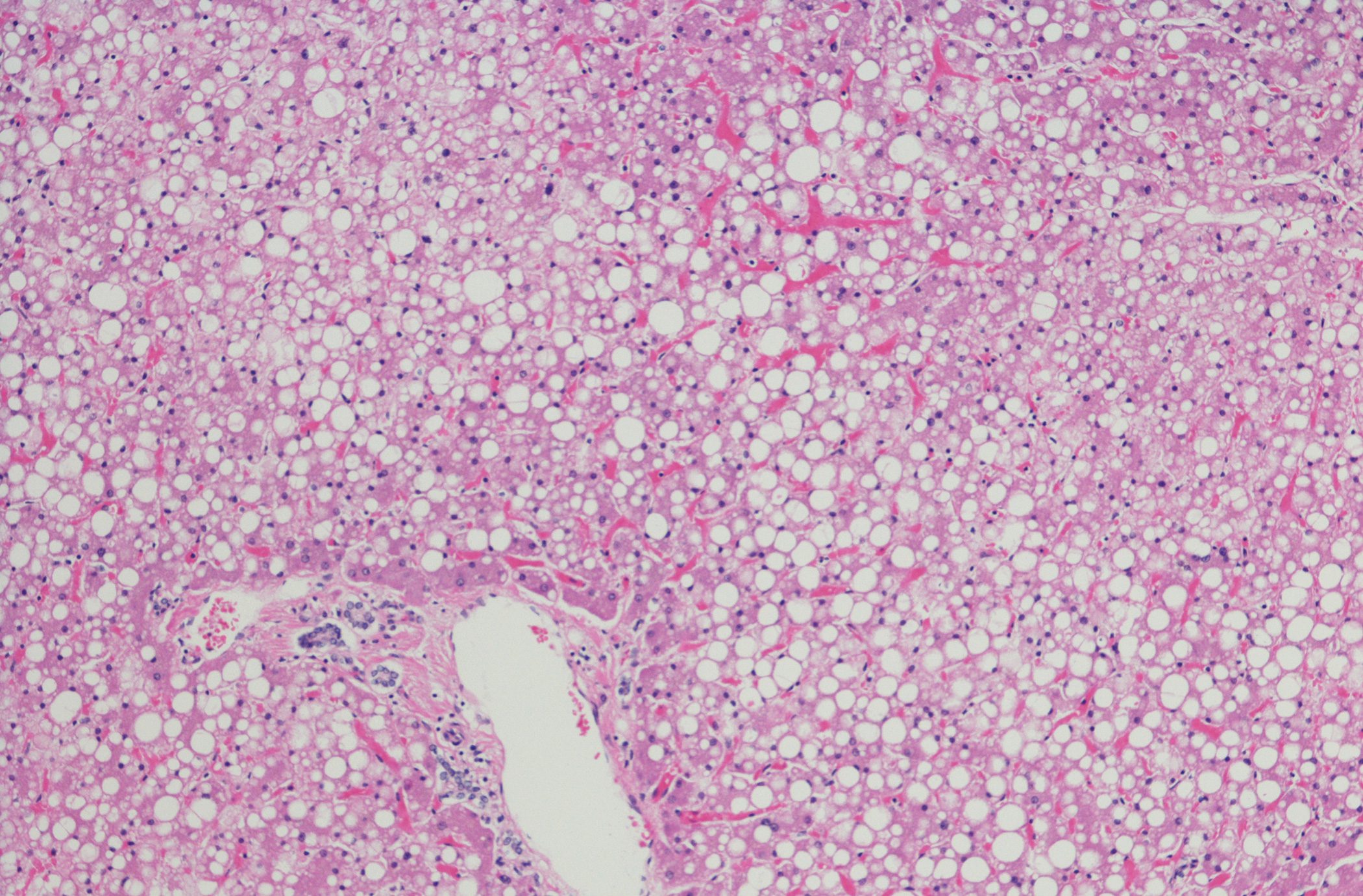
A typical feature of mastocytosis is the small-spotted maculopapular hyperpigmented skin lesions caused by mast cell infiltrates in the skin. Mechanical skin irritation reveals urticarial redness (Darier’s sign) [3,7]. However, a conspicuous skin finding is not present in all patients [8,9]. Elevated serum tryptase is present in almost all patients with systemic mastocytosis and is considered a screening parameter but is not sufficient for diagnosis. A normal value does not exclude systemic mastocytosis [4]. According to the 2016 WHO classification, a diagnosis of systemic mastocytosis can be made if the major criterion and one minor criterion or three of four minor criteria are met (Table 1)[4,8]. In addition to bone marrow biopsy (Fig. 1) and molecular genetic testing, osteodensitometry is useful once the diagnosis has been made [4]. In patients with gastrointestinal symptoms (e.g., diarrhea), colonoscopy with biopsy should be performed.
Treatment approaches: symptom-oriented vs. cytoreductive
In systemic mastocytosis, a distinction is made between indolent, “smouldering” and aggressive forms. The gender distribution is roughly balanced. Life expectancy is not limited in indolent systemic mastocytosis [9]. General measures include avoidance of triggers of mast cell degranulation, premedication with antihistamines if necessary, and an emergency kit for possible hymenopteran stings. For prophylaxis of mast cell degranulation-induced symptoms, regular use of a non-sedating H1 blocker (e.g., cetirizine or desloratadine) is recommended [3,4]. If gastrointestinal symptoms are present, an H2 blocker may also be considered (e.g., ranitidine). Glucocorticoids (e.g., budesonide) may be helpful for frequent anaphylactic reactions, diarrhea, malabsorption, and ascites [3,7]. An indolent form rarely progresses to aggressive systemic mastocytosis, and cytoreductive therapy may be required (Fig. 2)[3,4].
| Mast cells express the tyrosine kinase receptor KIT, which binds stem cell factor (SCF), a hematopoietic growth factor important for proliferation and differentiation, specifically promoting mast cell differentiation. An activating KIT D816 mutation (>95% KIT D816V) is detectable in 80-95% of SM patients and results in SCF-independent receptor activation with clonal expansion and tissue mast cell accumulation [16]. |
In advanced systemic mastocytosis: tyrosine kinase inhibitors.
Since the advent of tyrosine kinase inhibitors, KIT inhibition has become an attractive treatment approach [3,4]. Systemic imatinib is a tyrosine kinase inhibitor that can be used for the treatment of advanced systemic mastocytosis but is not effective in the presence of a Kit D816V mutation [3,4]. For rapidly progressive forms, the tyrosine kinase inhibitor midostaurin (Rydapt®) can be used [10]. Midostaurin demonstrated an overall response rate of 60% and a median overall survival of 28.7 months in advanced systemic mastocytosis with wild-type or kit D816V mutation in a phase II study [11]. The tyrosine kinase inhibitor reduced bone marrow mast cell density and serum tryptase levels, corrected organ damage, and attenuated splenomegaly[11]. Avapritinib is another tyrosine kinase inhibitor that has been shown to be effective in advanced systemic mastocytosis and has been approved by the US Food and Drug Administration (FDA) for this indication since 2021 [12]. Avapritinib is a highly potent selective inhibitor of Kit-D816V and the platelet-derived growth factor receptor PDGFRA. The efficacy of avapritinib was demonstrated in the Phase I EXPLORER study and in the Phase II PATHFINDER study [13,14]. The currently ongoing Phase II PIONEER trial is comparing the efficacy and safety of avapritinib versus placebo in patients with indolent or “smouldering” systemic mastocytosis [12,14]. Preliminary results show significant improvement in symptoms in patients with indolent systemic mastocytosis at 24 weeks compared with placebo (response rate was defined as ≥30% reduction in total symptom score) [12]. A daily dose of 25 mg showed good tolerability without serious side effects or discontinuation due to adverse reactions [12,14]. Primarily in aggressive forms of mastocytosis that do not respond to other therapies, polychemotherapy or the use of allogeneic stem cell transplantation may be considered[2,15].
Congress: SGAI Allergy and Immunology Update
Literature:
- Wagner N, Staubach P: Mastozytose – Pathogenese, Klinik und Therapie. JDDG 2018; 16(1): 42–59.
- Alvarez-Twose I, et al.: Management of adult mastocytosis. Expert Opinion on Orphan Drugs 2014; 2: 321–336.
- «Recent Developments in Management of Mastocytosis», Prof. Dr. med. Karin Hartmann, Allergy and Immunology Update, 27.–29.1.2023.
- Reinhart S, et al.: Anaphylaxieabklärung: Wenn Mastzellen krank machen. Swiss Med Forum 2019; 19(3132): 507–511.
- Valent P, et al.: Standards and standardization in mastocytosis: consensus statements on diagnostics, treatment recommendations and response criteria. European journal of clinical investigation 2007; 37: 435–453.
- Akin C: Mast cell activation disorders. J Allergy Clin Immunol Pract 2014; 2: 252–257.
- Horny HP, et al.: Die Mastozytose. Dtsch Arztebl 2008; 105(40): 686–692.
- Swerdlow SH, et al.: WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Revised 4th ed. International Agency for Research on Cancer; 2017.
- Theoharides TC, Valent P, Akin C: Mast cells, mastocytosis and related disorders. N Engl J Med 2015; 373(2): 163–172.
- Swissmedic: Information in brief, https://sai.refdata.ch,(last accessed 27.03.2023)
- Gotlib J, et al.: Efficacy and Safety of Literaturverzeichnis – 87 – Midostaurin in Advanced Systemic Mastocytosis. The New England journal of medicine 2016; 374(26): 2530–2541.
- «Beschwerdekontrolle bei Patienten mit Mastozytose unter aktuell verfügbaren medikamentösen Therapien», Le Huyen Tram Ho, Dissertation, 2022, https://refubium.fu-berlin.de/bitstream/handle/fub188/36476, (last accessed 27.03.2023)
- PATHFINDER Trial, www.blueprintmedicines.com/wp-content/uploads/2021/04/Blueprint-Medicines-AACR-2021-Avapritinib-Advanced-SM-PATHFINDER-Presentation.pdf, (last accessed 27.03.2023)
- PIONEER Trial, https://ir.blueprintmedicines.com/news-releases/news-release-details/blueprint-medicines-announces-positive-top-line-results-pioneer, (last accessed 27.03.2023)
- Vaes M, Benghiat FS, Hermine O: Targeted Treatment Options in Mastocytosis. Frontiers in medicine 2017; 4: 110.
- Mastozytose, www.onkopedia.com/de/onkopedia/guidelines/mastozytose-systemische/@@guideline/html/index.html, last accessed 27.03.2023)
- Valent P, et al.: Updated Diagnostic Criteria and Classification of Mast Cell Disorders: A Consensus Proposal. Hemasphere 2021 Oct 13;5(11): e646.
- Crupi, F, et al.: Histopathology and Molecular Genetics in Systemic Mastocytosis: Implications for Clinical Management. Int J Mol Sci 2022; 23: 8772. www.mdpi.com/1422-0067/23/15/8772, (last accessed 27.03.2023).
DERMATOLOGIE PRAXIS 2023; 33(3): 18-19 (published on 8.6.23, ahead of print)
InFo ONKOLOGIE & HÄMATOLOGIE 2023; 11(3): 38–39