Cardiogenic emboli are mostly embolized thrombi from the left atrial ear in atrial fibrillation. Primary and secondary prophylaxis of ischemic cerebral events is based on their causality, if this can be determined, and includes oral anticoagulation or corrective measures such as closure of a persistent foramen ovales, atrial septal defect, or left atrial appendage exclusion to avoid the long-term increasing complications of oral anticoagulation or a high risk of embolism in the absence of anticoagulation.
Stroke (cerebral infarction or apoplexy), which is about 85% ischemic, is the second leading cause of death after coronary heart disease, occurs in Western Europe with an annual incidence of about 250 / 100 000 persons and is the most frequent cause of permanent disability with often serious psychosocial and economic consequences. Mortality remains high at 25% (50% in patients under 65 years of age) despite medical advances in acute treatment over the last two decades. [1–3]. More than 80% of clinically apparent emboli involve the cerebral circulation, 80% of which involve the anterior (carotid-supplied) and 20% the posterior supply area.
The heart as a site of thrombus formation and thus source of emboli was first mentioned in a case report by Gowers in 1875 [4]. Imaging techniques such as transesophageal echocardiography (TOE), computed tomography with angiographic reconstruction, Doppler ultrasonography, and magnetic resonance imaging can document or at least highly suspect some of the sources of emboli in the current clinical context.
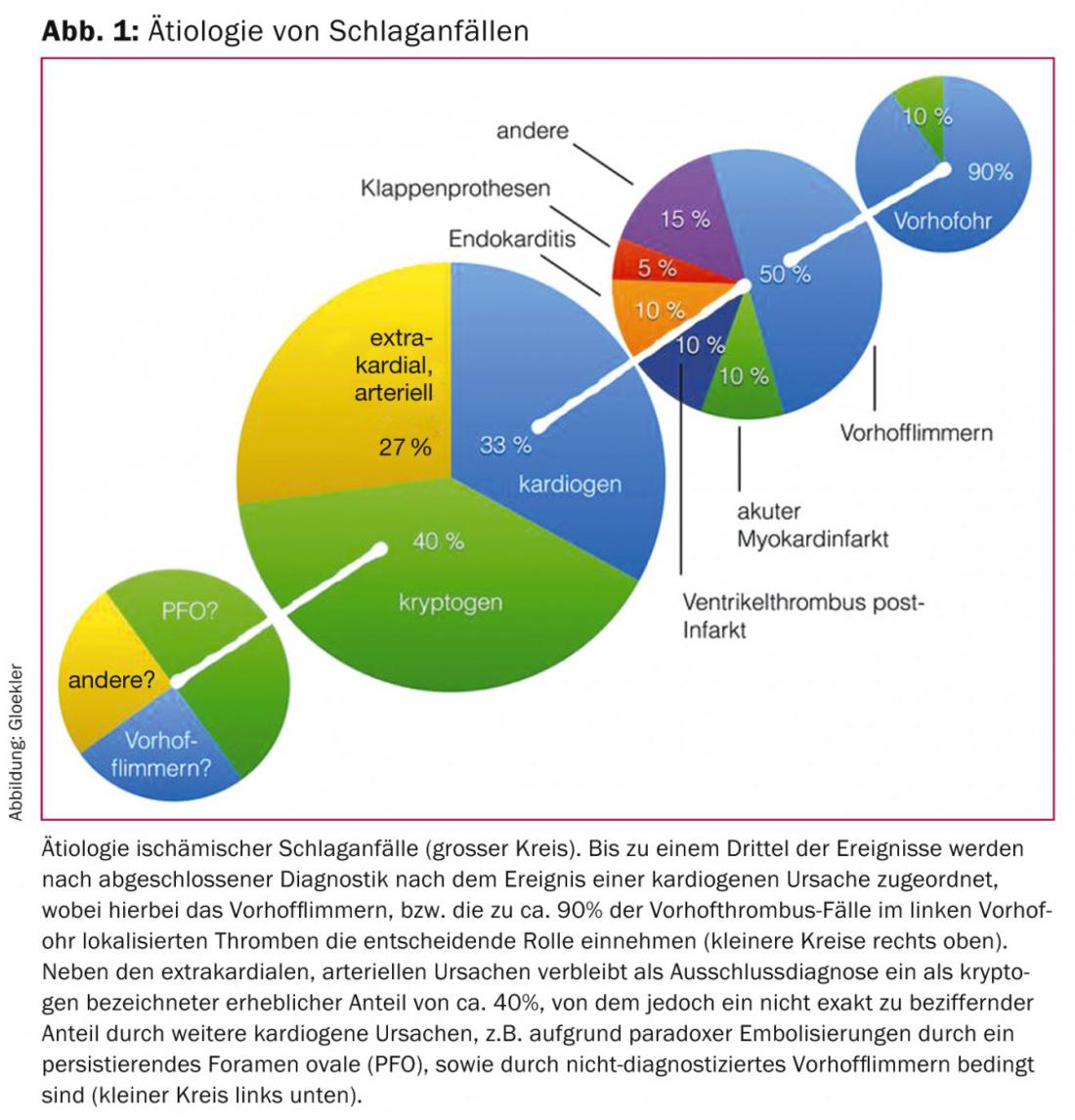
An underestimated proportion of all ischemic strokes (20-33%) due to the often impossible direct detection is referred to as cardiogenic-embolic. Among these, atrial fibrillation (VF) plays the most significant role (Fig. 1).
In VF, thrombus formation occurs in more than 90% of cases in the left atrial appendage, a blind-ended residuum of a primordial pulmonary vein.
In approximately 40% of all strokes, no objectifiable cardiac or extracardiac causes are found in the short window of diagnostic workup after an event. Such strokes are therefore often referred to as cryptogenic (unexplained) – but this category is overestimated. Cryptogenic strokes are not possible in principle and reflect our diagnostic weaknesses. For example, the presence of a persistent foramen ovale (PFO) or atrial septal defect (ASD) is rarely clarified and is not recognized as a conventional cause of stroke even in a positive case. Even unrecognized VF is rarely ruled out with ultimate consistency. Thus, the number of (trans)cardiac-related strokes is higher than generally assumed. If cerebral infarcts are present in different current areas, this suggests a nonbrain embolic source, and VF, PFO, and the nonassessable pulmonary veins come to the fore as embolic sources.
It is hoped that corrective preventive therapy can be improved and will be more widely used.
Atrial fibrillation, most common cause of cardiogenic embolism
Approximately 20% of all ischemic strokes are attributed to cardiogenic emboli caused by VF. VF is the most common arrhythmia with continued increase in prevalence and incidence (better detection and increasing average age of the population) during the last decades. Its prevalence is more than 7% in women and more than 10% in men over 80 years of age [5,6] (Fig. 1).
In two recently published studies that monitored the heart rhythm of patients after cryptogenic stroke compared with a standard Holter ECG using either an implantable recorder or a 30-day event recorder (CRYSTAL AF study [7] and EMBRACE study [8]), paroxysmal atrial fibrillation was documented to a significantly greater extent in patients with the prolonged recording method (12.4 vs. 2.0% during one year in CYSTAL AF, respectively. 16.1 versus 3.2% during three months in EMBRACE). As a result, significantly more patients in the groups with the improved monitoring received protection against recurrent stroke by initiation of oral anticoagulation (OAC). VF is underdiagnosed and thus undertreated in the context of source-searching for ischemic stroke.
Standard treatment with permanent anticoagulation
Both classic permanent OAK with vitamin K antagonists (VKA) or newer agents type non-vitamin K antagonism oral anticoagulation (NOAK) reduce the incidence of ischemic stroke and death by two-thirds and one-fourth, respectively, and are efficient in the prophylaxis of ischemic events [9–13]. However, in the case of classic OAK, there is significant underutilization in advance because of bleeding or increased risk of bleeding (e.g., fall risk) [14–16]. Thus, up to 44% of patients with AF receive no OAK at all as protection against embolic events [17]. In a large Swedish study from the National Stroke Registry of more than 21,077 patients after ischemic stroke in atrial fibrillation, the rates of treatment discontinuation for OAK were documented in five tertials during the first two years after apoplexy: After two years, instead of the initial 89% of patients, only 45% of patients were protected from apoplexy recurrence by OAK (Fig. 2) [18].
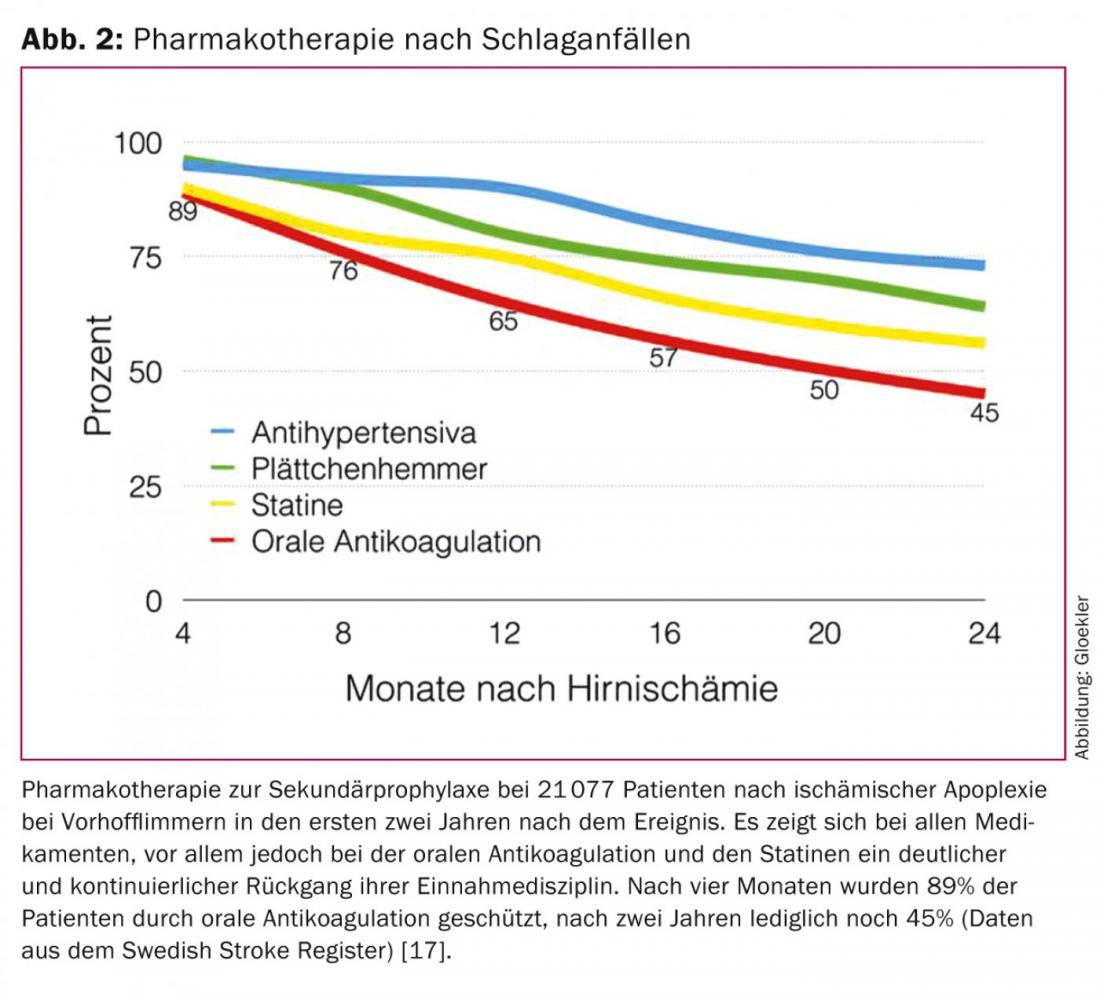
The problem of the high rate of treatment discontinuation for OAK is also known from other studies and clinical practice. As a further limitation, in those patients in whom OAK is continued, the INR (International Normalized Ratio) value is outside the therapeutic range in 30-46% of cases, with corresponding either inadequate embolic protection (INR <2) or increased risk of bleeding (INR >3) [13,19]. If an OAK can be administered permanently, the embolic protection has to be bought with annual rates for relevant bleeding of up to 15%, which tend to increase over the years [10–12].
In the large pivotal trials of the three NOAKs approved in Switzerland: RE-LY (“Dabigatran Randomised Evaluation of Long-Term Anticoagulation Therapy”) [10]ROCKET AF (“Rivaroxaban Once-daily, Oral, Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation”) and ARISTOTLE (“Apixaban for Reduction In Stroke and Other ThromboemboLic Events in Atrial Fibrillation”), as well as edoxaban, which is currently not yet approved in Switzerland: ENGAGE AF-TIMI 48 (“Edoxaban versus Warfarin in Patients with Atrial Fibrillation”) has consistently shown that NOAK is equivalent to or superior to classical OAK with regard to the prophylaxis of apoplexy. The risk of severe and especially life-threatening intracranial hemorrhage is significantly reduced with NOAK compared with classic OAK, but the rate of gastrointestinal bleeding is again slightly increased [10–13]. For the use of NOAKs, please refer to a recently published review article [20].
Despite the advantages of NOAK over classic OAK, a discontinuation rate of 17-27% has been documented in the individual studies even with NOAK, as well as an annual bleeding rate of 3.1 (RE-LY) to 14.4% (ROCKET AF), depending on the risk class of the different study populations. Another disadvantage, however overrated, is the lack to date of reliable means for rapid reversion of anticoagulation in the event of life-threatening hemorrhage.
Thus, even with NOAKs, it is in the nature of things that adequate anticoagulation always involves a certain rate of relevant bleeding.
Closure of the left atrial appendage as an alternative stroke prophylaxis
Due to the fact, which has been well documented for some time from pathological, surgical and imaging studies, that more than 90% of all thrombi in VF originate from the blind sac of the left atrial appendage (LVO), as well as the risks of systemic OAK and NOAK ad infinitum, the exclusion of the LVO from the circulation by surgical or catheter-based occlusion is the obvious choice [21,22]. The surgical method was first published by Madden in 1949 [23], but did not gain general acceptance because of incomplete exclusion results and lack of comparative studies.
The efficacy and safety of the concept of a single intervention instead of permanent anticoagulation to achieve the common goal of efficient stroke prophylaxis with few side effects could be demonstrated for the first time in a randomized fashion in the PROTECT AF study [24] and is supported by the recently published PREVAIL trial, which was also randomized. [25] substantiated: a one-time VHO closure with the WATCHMAN system is at least competitive with permanent OAK with warfarin. From currently unpublished data with an extended observation period from the PROTECT AF trial, it is logical to assume superiority of catheter-based VHO closure over continued anticoagulation, because the net benefit of VHO closure compared with continued anticoagulation increases with increasing duration after intervention. Interestingly, this net benefit was most pronounced in high-risk patients [26]. For the Amplatzer Cardiac Plug, which compared with the cap-like configuration of the WATCHMAN system has a body occluding the VHO lumen and a disc sealing the ostium of the VHO, is made of nitinol, and is implanted according to the pacifier principle, similar data exist from smaller nonrandomized studies (figs. 3 and 4) [27–30].
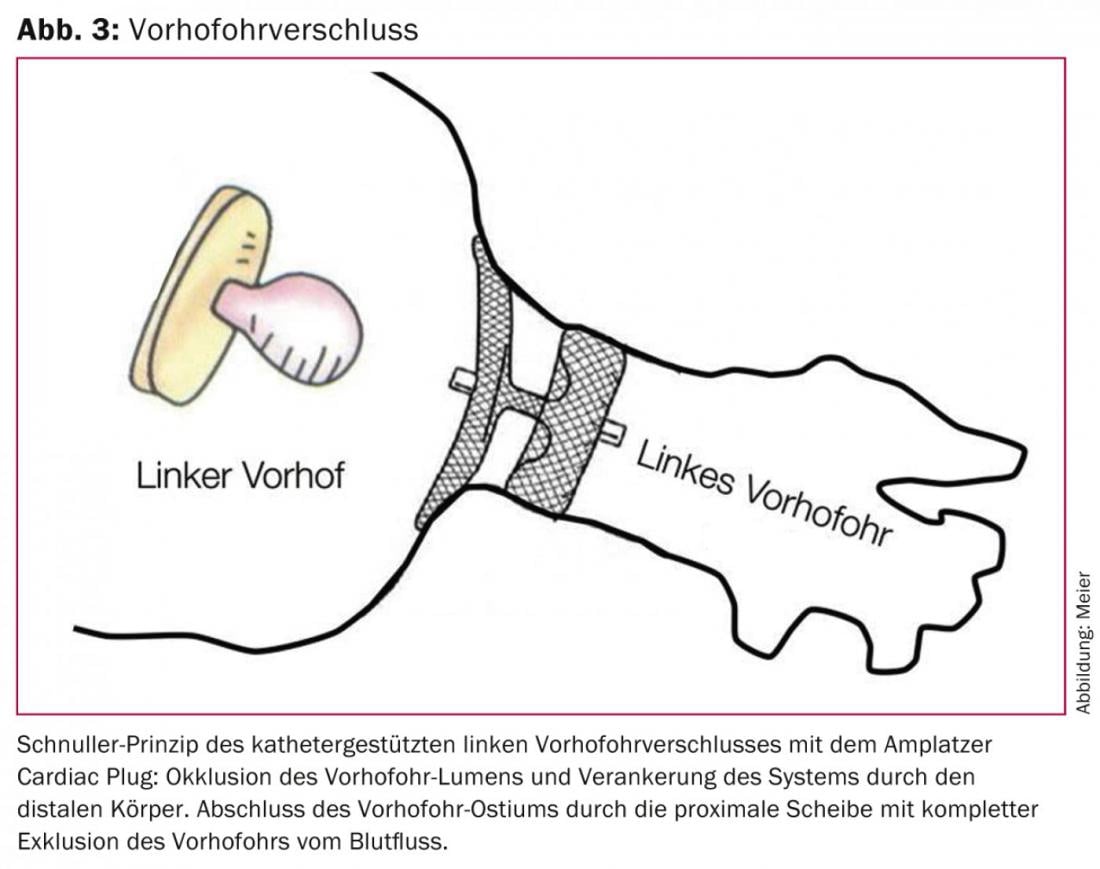
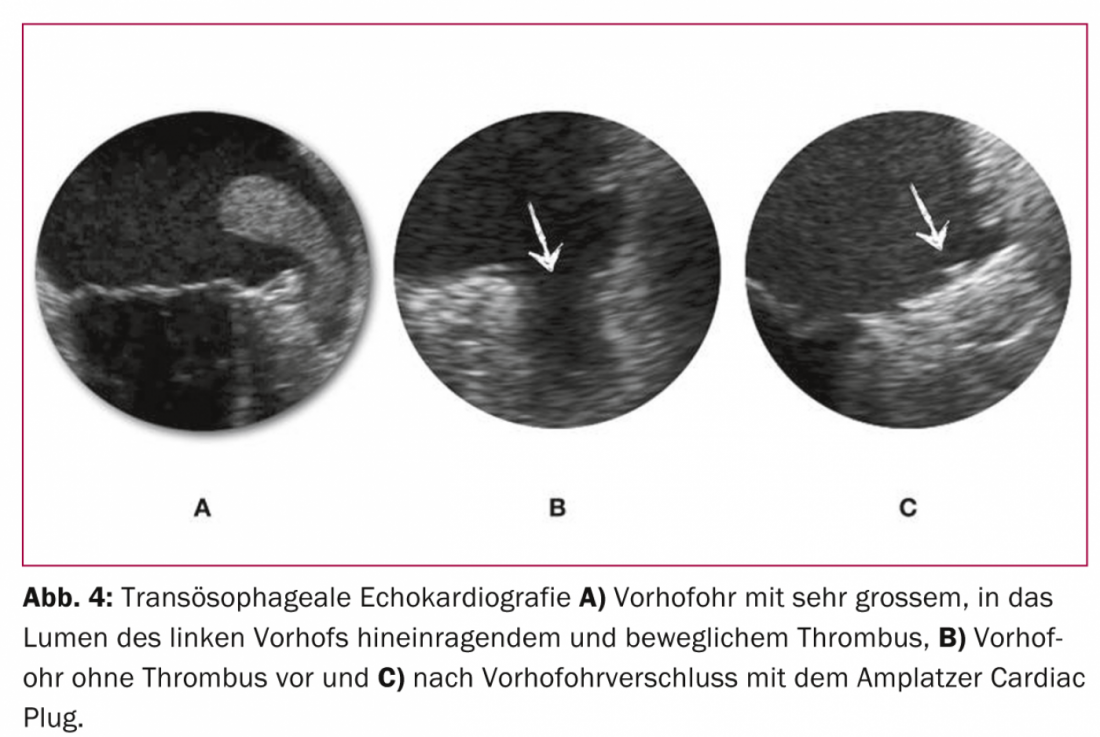
Like any intervention, VHO closure has a certain complication rate. In currently more than 400 patients treated so far in our center with the Amplatzer system and without toe or general anesthesia, the combined safety endpoint of relevant complications (death, procedural stroke, pericardial tamponade, or need for surgical revision) is 8.5%. Contrast this with an annual rate of major bleeding of approximately 4% with rivaroxaban in the ROCKET AF trial [11], which studied patients at comparable risk for cerebrovascular events and bleeding. Basically, then, the anticipated rates of one-time periprocedural complications of VHO closure must be weighed against those of cumulative complications of (N)OAK. Thus, with an approximate residual life expectancy of three years or longer, a net clinical and prognostic benefit of VHO closure over permanent anticoagulation can be assumed. This is confirmed by not yet published data from the PROTECT AF trial with first time evidence of significantly reduced all-cause mortality in favor of VHO closure.
Based on these data, catheter-based VHO closure is an equivalent alternative to permanent anticoagulation in the medium term and superior in the longer term. Comparative studies with NOAK will have to wait. Based on the proven efficacy of VHO closure and the limitations that continue to exist with NOAKs, it can be assumed that VHO closure is fundamentally competitive or even superior to NOAKs because of the conceptual differences in the prophylaxis of cerebral strokes that have been demonstrated.
Although catheter-based radiofrequency ablation is a valid therapy to control symptoms, it is not curative and rarely saves stroke prophylaxis by (N)OAK or catheter-based VHO occlusion permanently. Thus, it corresponds to a valuable addition to stroke prophylaxis rather than an alternative [31].
Paradoxical emboli due to persistent foramen ovale or atrial septal defect
An association between cryptogenic strokes and PFO was described as early as 1877 by Julius Conheim and later by numerous others [32]. The search for a PFO is part of the standard diagnostic workup after a cerebrovascular event in all patients and especially in younger patients who do not yet have atherosclerosis [33]. Compared with the much rarer ASD, which represent true structural defects in the septum, the PFO is a residual slit or tunnel resulting from incomplete fusion of the two parts of the interatrial septum in the postnatal period. Following the publication of the three randomized trials CLOSURE I (“Evaluation of the STARFlex Septal Closure System in Patients with a Stroke and/or Transient Ischemic Attack due to Presumed Paradoxycal Embolism through
a Patent Foramen Ovale”) [34], PC (“Clinical Trial Comparing Percutaneous Closure of patient Foramen Ovale Using the Amplatzer PFO Occluder with Medical Treatment in Patients with Cryptogenic Embolism”). [35] and RESPECT (“Randomized Evaluation of Recurrent Stroke Comparing PFO Closure to Established Current Standard of Care Treatment”). [36]The situation remains controversial in view of the inherent limitations of each study, such as the type of closure system, the number of patients, and the length of the observation period. These studies failed to demonstrate significant superiority of PFO closure over medical therapy [34–38]. However, at least in subgroups with large shunts, septal aneurysms, or coincidence of structures such as a valvula eustachii or Chiari networks, superiority of PFO closure over medical therapy can be assumed. In terms of procedure safety, the Amplatzer system is superior to the Starflex system tested in the CLOSURE I study [39]. Due to the small number of events in all three randomized trials, they lack the statistical power assumed in their design: for example, in the CLOSURE I trial, of 909 patients, 5.5 vs. 6.8% (p=0.37) achieved the combined endpoint of stroke, transient ischemic attack (TIA), and systemic embolism during a 2-year follow-up period, and 2.9 vs. 3.1% (p=0.79) achieved the endpoint of stroke alone [34]. In the PC study, another prospective and randomized study of 414 patients, which, like the RESPECT study, performed PFO closure with the Amplatzer systems (Fig. 5) compared with drug therapy over a follow-up period of two to four years, 3.4 (n=7) versus 5.2% (n=11) met the combined endpoint of death, stroke, TIA, or systemic embolism (p=0.34) [35]. In the RESPECT study, 980 patients were randomized and followed up for 2.5 years: Nine patients in the occlusion group versus 16 patients in the drug group experienced a cerebrovascular event during this period (p=0.08) [36]. There will be further data collection in these two studies, and it is likely that with an extended follow-up period, the previous trend in favor of PFO closure with the Amplatzer system will change to a significant difference.
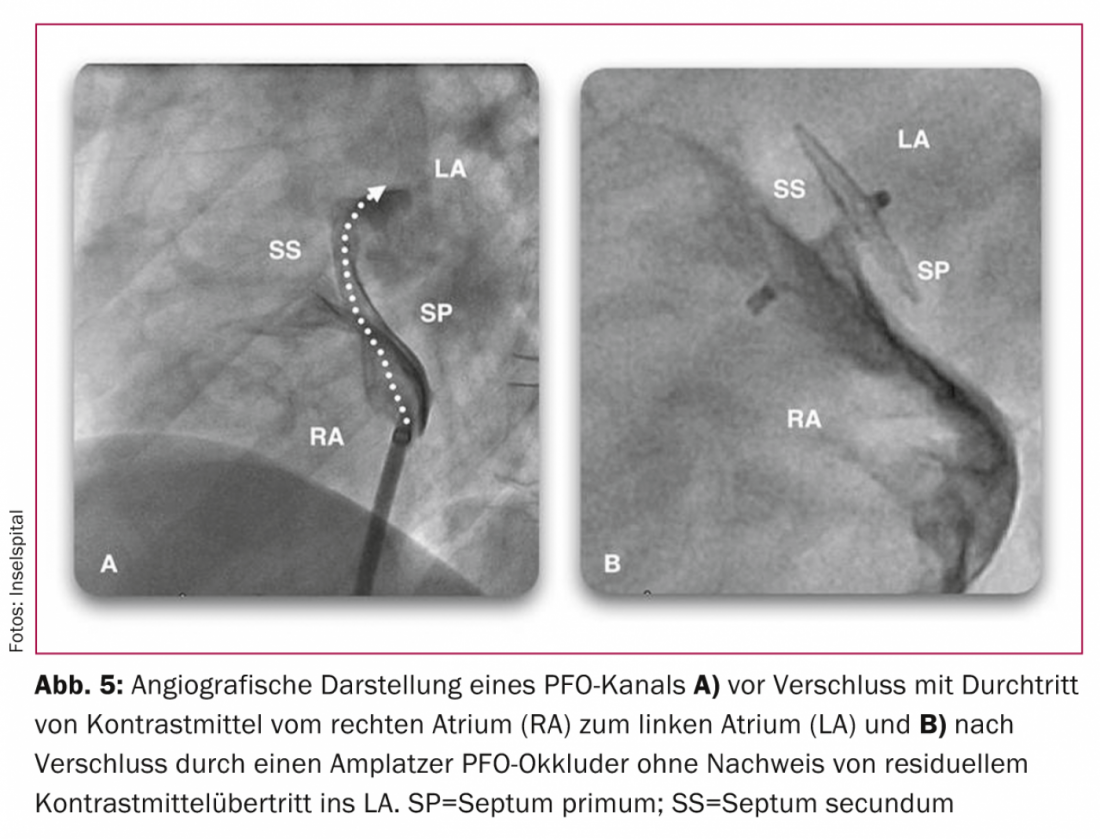
Consistent with the expected results of the next data collection from the PC and RESPECT trials, in a long-term observational study comparing the two groups using propensity matching, with a mean observation period of nine years, PFO closure was shown to be superior to drug therapy in 308 patients with respect to achieving the combined endpoint (11 vs. 21%, p=0.033). Even a significant survival benefit of PFO closure was shown when comparing years of observation with or without PFO closure [40]. A meta-analysis showed an 84 percent risk reduction for recurrent neurologic events (one event versus five per 100 patient-years) and a significant difference in favor of PFO closure in elderly patients and those with atrial septal aneurysms or thrombophilia [41].
Due to the relatively small number of cases with still insufficient follow-up duration, the low number of neurological events, and varying quality of the closure systems, the three large studies mentioned individually do not yet show a clear significant superiority of PFO closure over drug-only therapy, but they do show a solid trend in this direction. Thus, it can be assumed that in the medium term, evidence from extended observation time of previous studies, meta-analyses, or new and more conclusive studies will demonstrate superiority of PFO closure in secondary prevention of ischemic cerebrovascular events. PFO closure, which is technically easy to perform with an excellent success rate of nearly 100% and virtually no complications, should be available to every patient for secondary prophylaxis in terms of mechanical inoculation, especially those of younger age with septal aneurysms or larger shunts, based on the overall view of current data, given the potentially catastrophic effects of any cerebrovascular event. In addition to some useful side effects such as reduction of migraine, sleep apnea, or even myocardial infarction due to paradox-embolic coronary occlusions, this may avoid lifelong anticoagulation, which is cumulatively associated with high risks. To prevent a stroke over 20 years, only nine PFO closures need to be performed.
Other sources of cardiogenic embolism
In addition to atrial fibrillation and PFO, cardiac thrombi in acute myocardial infarction or as a late sequela after transmural myocardial infarction, emboli in mitral valve stenosis, sick sinus syndrome, atrial myxomas, and thrombotic deposits on mechanical or biological valve prostheses or septic emboli in endocarditis are rare causes of apoplexy (Fig. 1).
Depending on the severity of the findings, the first stage of treatment is the initiation of oral anticoagulation, followed by follow-up imaging studies. Secondarily, surgical removal should be considered in the absence of thrombus resolution, floating clots, tumors, or florid endocarditis.
The entire venous pulmonary vein bed represents a nonvisible potential source of embolic cerebral insults. It should increasingly become the focus of clinical studies in this regard.
PD Dr. med. Steffen Gloekler
Potential conflicts of interest: Bernhard Meier has received institutional research support and speaking fees from St. Jude Medical.
Literature:
- Go AS, et al. (American Heart Association Statistics Committee and Stroke Statistics Subcommittee): Heart disease and stroke statistics – 2014 update: a report from the American Heart Association. Circulation 2014; 129: e28-e292.
- Lozano R, et al: Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2095-2128.
- Feigin VL, et al. (Global Burden of Diseases, Injuries, and Risk Factors Study 2010 [GBD 2010] and the GBD Stroke Experts Group): Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 2014; 383: 245-254.
- Gowers W: On a case of simultaneous embolism of central retinal and middle cerebral arteries. Lancet 1875; 2: 794.
- Miyasaka Y, et al: Mortality trends in patients diagnosed with first atrial fibrillation: a 21-year community-based study. J Am Coll Cardiol 2007; 49: 986-992.
- Go S: Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors In Atrial Fibrillation (ATRIA) Study. J Am Med Assoc 2001; 285: 2370-2375.
- Gladstone DJ, et al. (EMBRACE Investigators and Coordinators): Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014; 370: 2467-2477.
- Sanna T, et al. (CRYSTAL AF Investigators): Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014; 370: 2478-2486.
- Hart RG, Pearce LA, Aguilar MI: Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med 2007; 146: 857-867.
- Connolly SJ, et al. (RE-LY Steering Committee and Investigators): Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361: 1139-1151.
- Patel MR, et al. (ROCKET AF Investigators): Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011; 365: 883-891.
- Granger CB, et al. (ARISTOTLE Committees and Investigators): Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2011; 365: 981-992.
- Giugliano RP, et al. (ENGAGE AF-TIMI 48 Investigators): Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 2013; 369: 2093-2104.
- Casciano JP, et al: The costs of warfarin underuse and nonadherence in patients with atrial fibrillation: a commercial insurer perspective. J Manag Care Pharm 2013; 19: 302-316.
- Gladstone DJ, et al: Potentially preventable strokes in high-risk patients with atrial fibrillation who are not adequately anticoagulated. Stroke 2009; 40: 235-240.
- Wrigley BJ, Lip GY: Can the WATCHMAN device truly PROTECT from stroke in atrial fibrillation? Lancet Neurol 2009; 8: 877-878.
- Waldo AL, et al. (NABOR Steering Committee): Hospitalized patients with atrial fibrillation and a high risk of stroke are not being provided with adequate anticoagulation. J Am Coll Cardiol 2005; 46: 1729-1736.
- Glader EL, et al: Persistent use of secondary preventive drugs declines rapidly during the first 2 years after stroke. Stroke 2010; 41: 397-401.
- Onalan O, et al: Nonpharmacologic stroke prevention in atrial fibrillation. Expert Rev Cardiovasc Ther 2005; 3: 619-633.
- Steffel J: New oral anticoagulants – 10 questions from daily use. Cardiovasc 2014; 13: 12-16.
- Blackshear JL, Odell JA: Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 1996; 61: 755-759.
- Di Biase L, et al: Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol 2012; 60: 531-538.
- Madden JL: Resection of the left auricular appendix; a prophylaxis for recurrent arterial emboli. J Am Med Assoc 1949; 140: 769-772.
- Holmes DR, et al. (PROTECT AF Investigators): Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet 2009; 374: 534-542.
- Holmes DR, et al: Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients With Atrial Fibrillation Versus Long-Term Warfarin Therapy. J Am Coll Cardiol 2014; 64: 1-12.
- Gangireddy SR, et al: Percutaneous left atrial appendage closure for stroke prevention in patients with atrial fibrillation: an assessment of net clinical benefit. Eur Heart J 2012; 33: 2700-2708.
- Park JW, et al: Left atrial appendage closure with Amplatzer Cardiac Plug in atrial fibrillation: initial European experience. Catheter Cardiovasc Interv 2011; 77: 700-706.
- Nietlispach F, et al: Amplatzer left atrial appendage occlusion: Single center 10-year experience. Catheter Cardiovasc Interv 2013; 82: 283-289.
- Nietlispach F, et al: Percutaneous left atrial appendage closure. Europ Geriat Med 2012; 3: 308-311.
- Nietlispach F, et al: Ad hoc percutaneous left atrial appendage closure. J Invasive Cardiol 2013; 25: 683-686.
- Calkins H, et al: 2012 HRS/EHRA/ECAS Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 2012; 14: 528-606.
- Lechat P, et al: Prevalence of patent foramen ovale in patients with stroke. N Engl J Med 1988; 318: 1148-1152.
- Meier B, et al: Secondary stroke prevention: patent foramen ovale, aortic plaque, and carotid stenosis. Eur Heart J 2012; 33: 705-713, 713a, 713b.
- Furlan AJ, et al. (CLOSURE I Investigators): Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med 2012; 366: 991-999.
- Meier B, et al. (PC Trial Investigators): Percutaneous closure of patent foramen ovale in cryptogenic embolism. N Engl J Med 2013; 368: 1083-1091.
- Carroll JD, et al. (RESPECT Investigators): Closure of patent foramen ovale versus medical therapy after cryptogenic stroke. N Engl J Med 2013; 368: 1092-1100.
- Spencer FA, et al: Systematic review of percutaneous closure versus medical therapy in patients with cryptogenic stroke and patent foramen ovale. BMJ Open 2014; 4: e004282.
- Meier B: Patent foramen ovale, is it more dangerous to close it than lo leave it open? Cardiovasc Med 2012; 15: 183-185.
- Messé SR, Kent DM: Still no closure on the question of PFO closure. N Engl J Med 2013; 368: 1152-1153.
- Wahl A, et al: Long-term propensity score-matched comparison of percutaneous closure of patent foramen ovale with medical treatment after paradoxical embolism. Circulation 2012; 125: 803-812.
- Agarwal S, et al: Meta-analysis of transcatheter closure versus medical therapy for patent foramen ovale in prevention of recurrent neurological events after presumed paradoxical embolism. JACC Cardiovasc Interv 2012; 5: 777-789.
CARDIOVASC 2014; 13(4): 3-8