According to the newly issued ESPEN guidelines, enteral nutrition measures should generally be preferred to parenteral nutrition. There are some exceptions, however, and these include contraindications such as bowel obstruction, severe shock, intestinal ischemia, high-output fistulas, or intestinal bleeding.
In these cases, parenteral nutrition may be needed for a period of days or weeks until gastrointestinal function is restored, according to new guidelines from the European Society for Clinical Nutrition and Metabolism (ESPEN) published in the journal Clinical Nutrition in March of this year. [1,2] (Overview 1). In the presence of acute intestinal failure/enterocutaneous fistulae, parenteral nutrition is often required due to gastrointestinal tract impairment, possibly in addition to enteral nutrition [3]. A combination of enteral and parenteral nutrition should be considered in patients in whom more than 60% of the required energy intake is not provided by enteral measures (recommendation 25A) [2]. Parenteral infusion of fluid and electrolytes may be required if high-output stomas persist (Recommendation 9B). In the perioperative period in patients with IBD, parenteral nutrient supplementation is usually indicated in addition to enteral nutrition (Recommendation 25B) [1]. In Crohn’s disease patients with persistent gastrointestinal failure (such as short bowel syndrome secondary to resection), nutrient infusion is a necessary and life-saving measure, at least in the early stages of intestinal failure (recommendation 26B) [1]. Promptly following proctocolectomy or colectomy, water and electrolyte supplementation is required to ensure hemodynamic stability (Recommendation 27B) [1].

Stoma patients benefit from parenteral nutrition
Persistent and severe diarrhea or a high-output stoma can lead to intestinal insufficiency characterized by malabsorption, unintentional weight loss, malnutrition, and/or dehydration. Malabsorption is an important factor for malnutrition in CED [4,5]. In a retrospective study [6] of 687 ostomy patients, it was shown that early high-output of an ileostomy within three weeks is common and although 49% spontaneously remit, 51% require further medical treatment, mostly due to a previous short bowel syndrome. 71% of patients were treated with oral hypotonic fluid restriction, glucose-salt solution, and anti-diarrheal medication to discontinue parenteral infusions. In 8% of cases, parenteral or subcutaneous saline infusion had to be continued at home. It was shown several years ago that treatment with oral fluid intake and monitoring of urinary sodium is feasible in the home setting [7].
In a study of 13 adults with high-output stomas, oral rehydration solutions containing rice maltodextrin supplements improved sodium and potassium balance. An association of increased body weight with decreased serum renin concentrations suggests that water balance could also be achieved [11]. In another study, three different saline and/or glucose solutions were tested in six patients with jejunostomies. Based on the results in this relatively small sample, an orally ingested glucose-electrolyte solution appears to be an adequate substitute for sodium in patients with high-output stomas [8].
In case studies, treatment with hypotonic fluid restriction, sodium-enriched diet, enteral-only nutrition, and/or parenteral sodium-containing infusions showed beneficial effects in Crohn’s disease patients with high-output stoma.
Following surgical intervention, early postoperative nutrition has been shown to be associated with a significant reduction in complications compared with traditional postoperative nutrition methods. No negative effect on mortality, dehiscence of the anastomosis, resumption of bowel function, or hospitalization duration was found [9]. In a Cochrane Systematic Review [10], early oral or enteral feeding, including clear liquids on the first or second day after surgery, did not worsen healing of colonic or rectal anastomoses and was correlated with significantly less hospitalization time.
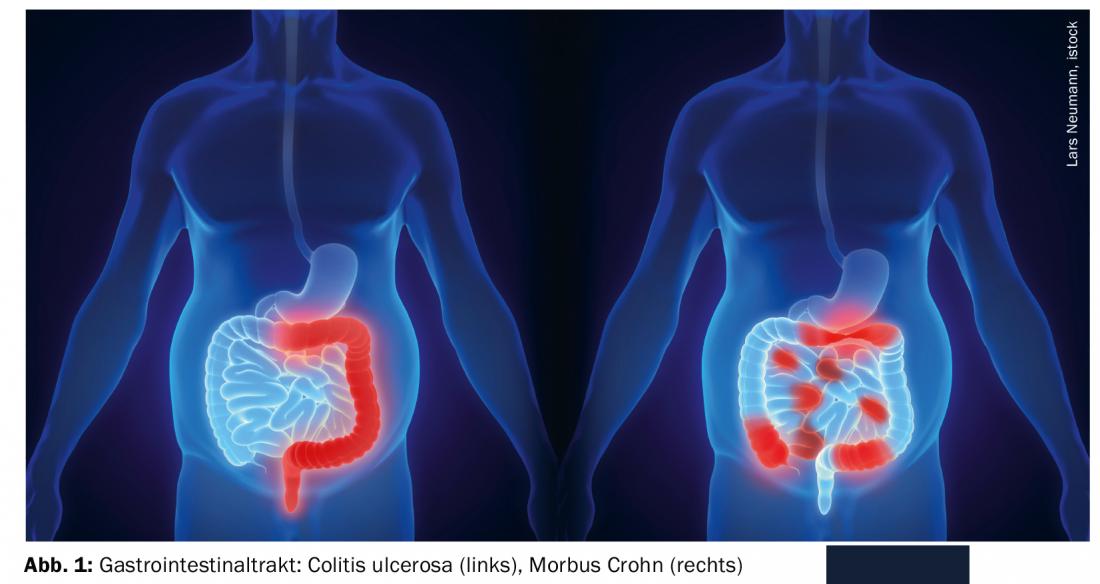
General nutrition-related measures in CED.
The ESPEN guidelines recommend that patients with inflammatory bowel disease who are at increased risk for malnutrition should be screened for malnutrition at the time of diagnosis and during the course of their disease. If malnutrition is identified, adequate treatment should be provided, as quality of life, complications, prognosis, and mortality may be affected. In active IBD, protein intake should be increased (up to 1.2-1.5 g/kg/d). In the remission phase, protein requirements are usually not increased; here, an intake of 1 g/kg/d is sufficient, analogous to healthy adults. All patients with IBD should be regularly screened for micronutrient deficiencies, and specific deficiencies in vitamins or trace elements should be addressed.
Testing for anemia is also important. If iron deficiency anemia is identified, treatment with iron supplementation is recommended to replenish hemoglobin (Hb) levels and iron stores. In mild anemia and clinically inactive IBD, oral iron is recommended as first-line treatment in the absence of contraindication/intolerance. Intravenous iron replacement therapy is recommended in patients with clinically active IBD or intolerance to oral iron, as well as in Hb levels below 100 g/L and in patients requiring erythropoietin-stimulating agents.

There is no specific IBD diet that has been shown to promote remission. In adult and pediatric patients with active IBD disease and those being treated with steroids, calcium levels and 25(OH) vitamin levels should be monitored and supplemented as necessary to prevent low bone density. If osteopenia or osteoporosis is present, it is recommended to treat this according to the appropriate guidelines. Exclusion diets are not recommended; there is no evidence that this promotes remission of active IBD, even when a patient suffers from individual intolerances. When should probiotics be used? In patients with mild to moderate ulcerative colitis, Lactobacillus reuteri or “VSL#3” may be considered to induce remission, but no other probiotics. Probiotics should not be used in active ulcerative colitis.
Literature:
- Bischoff SC, et al: ESPEN practical guideline: Clinical Nutrition in inflammatory bowel disease. ESPEN. Clin Nutr 2020; 39(3): 632-653.
- Weimann A, et al: ESPEN guideline: clinical nutrition in surgery. Clin Nutr 2017; 36: 623-650
- Slonim AE, et al: Effect of exclusion diet with nutraceutical therapy in juvenile Crohn’s disease. J Am Coll Nutr. 2009; 28: 277-285.
- Pironi L, et al: Home artificial nutrition & chronic intestinal failure; acute intestinal failure special interest groups of ESPEN. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr 2015; 34: 171-180.
- Hart JW, et al: Measured versus predicted energy expenditure in children with inactive Crohn’s disease. Clin Nutr 2005; 24: 1047-1055.
- Baker ML, et al: Causes and management of a high-output stoma. Colorectal Dis. 2011; 13: 191-197
- Grischkan D, et al: Maintenance of home hyperalimentation in patients with high-output jejunostomies. Arch Surg 1979; 114: 838-841.
- Nightingale JM, et al: Oral salt supplements to compensate for jejunostomy losses: comparison of sodium chloride capsules, glucose electrolyte solution, and glucose polymer electrolyte solution. Gut 1992; 33: 759-761.
- Osland E, et al: Early versus traditional postoperative feeding in patients undergoing resectional gastrointestinal surgery: a meta-analysis. J Parenter Enter Nutr 2011; 35: 473-448.
- Shukla HS, et al: Enteral hyperalimentation in malnourished surgical patients. Indian J Med Res 1984; 80: 339-346.
- Pironi L, et al: Oral rehydration solution containing rice maltodextrins in patients with total colectomy and high intestinal output. Int J Clin Pharmacol Res 2000; 20: 55-60.
HAUSARZT PRAXIS 2020; 15(11): 40-41