Pain is one of the most difficult symptoms to detect. Many other signs of disease, such as skin symptoms or paralysis, are visible, palpable, and thus objectifiable. In the assessment of pain, on the other hand, we are entirely dependent on the description of the affected person; the symptom always remains subjective.
Pain is one of the most difficult symptoms to detect. Many other signs of disease, such as skin symptoms or paralysis, are visible, palpable, and thus objectifiable. In the assessment of pain, on the other hand, we are entirely dependent on the description of the affected person; the symptom always remains subjective.
Definition
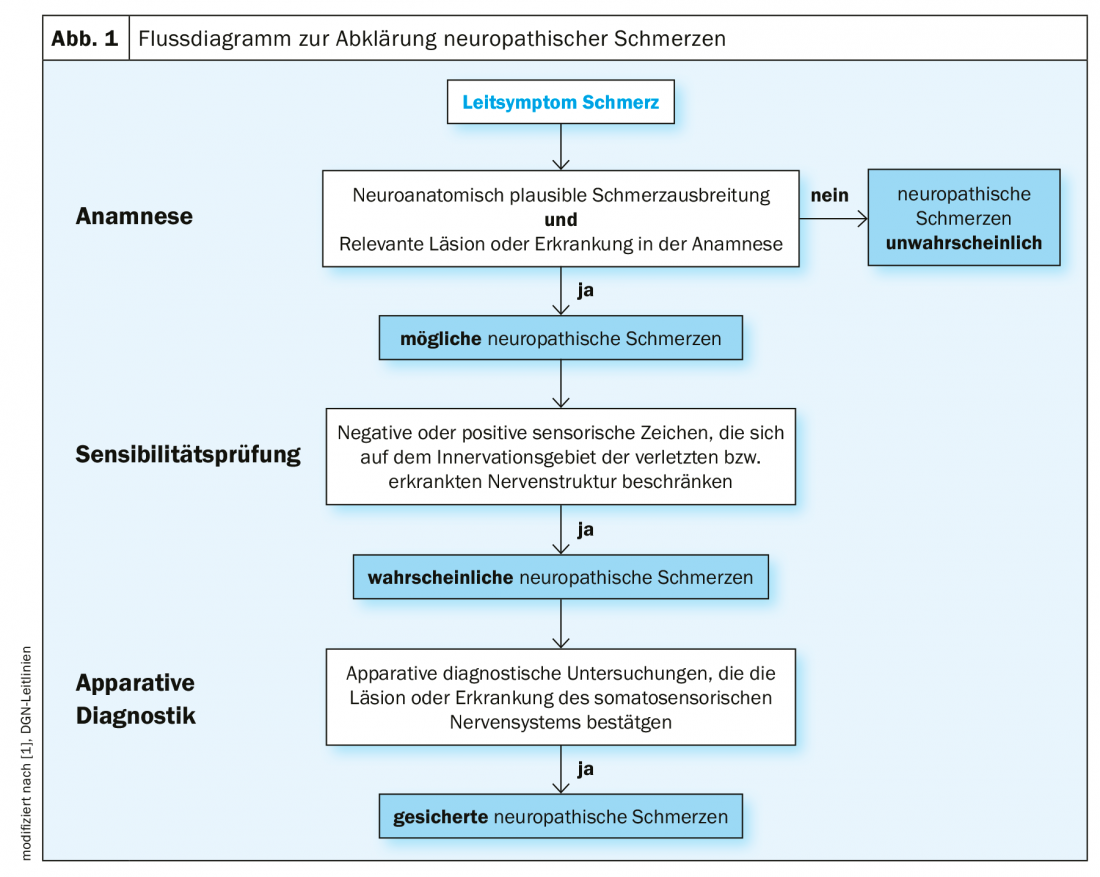
Neuropathic pain is defined as “pain that occurs as a direct result of damage or lesion to the somatosensory system” [1,2]. This definition requires evidence of such damage as an inescapable condition for the diagnosis of “neuropathic pain.” In other words, the patient’s information is not sufficient to establish the diagnosis as confirmed. Depending on the evidence of such damage, the classification is made into definite, probable, possible and improbable neuropathic pain [3] (Fig. 1) . Relevant damage to the somatosensory system can be detected by means of special examination procedures, which include quantitative sensory testing (QST).
Background
The QST examination is a standardized extension of neurological sensitivity testing and provides a comprehensive analysis of somatosensory nervous system function [4]. It is performed according to the protocol of the German Neuropathic Pain Research Network (DFNS). QST complements other existing neurological and electrophysiological measurement techniques such as neurography. While the latter examines the function of the thicker nerve fibers, QST additionally records the function of the thin, little to non-myelinated nerve fibers. We know from post-mortem studies that the proportion of small-caliber nerve fibers in the peripheral nervous system is approximately 80% [5,6].
Thin nerves – big effect
The thin nerve fibers play a particularly important role in diseases associated with reduced sensation of heat, cold or touch, as well as in pain. The free nerve endings are located in the skin area, receive stimuli and convert them into electrical potentials [7]. Lesions in the peripheral or central nervous system are often accompanied by increased pain sensitivity of the skin, “plus symptoms” (plus signs: hyperalgesia and allodynia), and/or decreased perception, “negative symptoms” (minus signs: hypesthesia, hypalgesia). Nonspecific sensations with/without pain can manifest as initial symptoms of a systemic disease that is still latent in terms of laboratory chemistry, such as diabetes mellitus. These symptoms characterize neurobiological mechanisms involved in the development of neuropathic pain. QST allows the functional state of all primary afferent fiber systems forming the somatosensory system to be recorded [8]. These include the Aβ-nerve fibers, which are thickly myelinated, large in diameter, and rapidly conduct stimuli (proprioception, light touch) to the thalamus via the posterior cord tracts and lemniscal system. The sparsely myelinated Aδ-fibers and the unmyelinated C-fibers slowly conduct nociceptive and thermal stimuli to the thalamus via the anterior cords and the tractus spinothalamicus. While the Aδ-fibers are responsible for the first pain, which is bright, sharp, and easily localized, the C-fibers are responsible for the second, sustained pain [9].
A standardized procedure
As mentioned above, the QST examination is performed according to the standardized protocol of the German Neuropathic Pain Research Network (DFNS) [4]. The validity, reliability (test-retest and interobserver reliability) and quality assurance possibilities of the measurement method have been evaluated in multicenter studies in both patients and healthy volunteers, and a normative value database has been created [10,11]. Age- and sex-related standard values are available for hand, foot, face and back [10,12]. For the validation of the QST method, a measuring station was established where all QST parameters are examined. This measuring station is used as part of QST training courses to train the exact application of QST stimuli and contributes to quality assurance.
Operating principle
The standardized QST battery of the DFNS consists of 7 individual tests, in which a total of 13 parameters are recorded. The tests are performed exclusively on the skin and a test area and a mirror image control area are examined so that a complete sensory profile can be obtained within one hour [4]. This involves applying calibrated stimuli to the skin to determine perceptual, pain or pain tolerance thresholds. This allows complete recording of the function of all somatosensory nociceptive and non-nociceptive submodalities (plus sign – increase in function [Hyperalgesie] or minus sign – [loss of function, hypesthesia]). Precise instructions for the investigator with standardized wording are available. The same calibrated thermal and mechanical test stimuli are always applied in the same test sequence, starting with the thermal test [4,13,14].
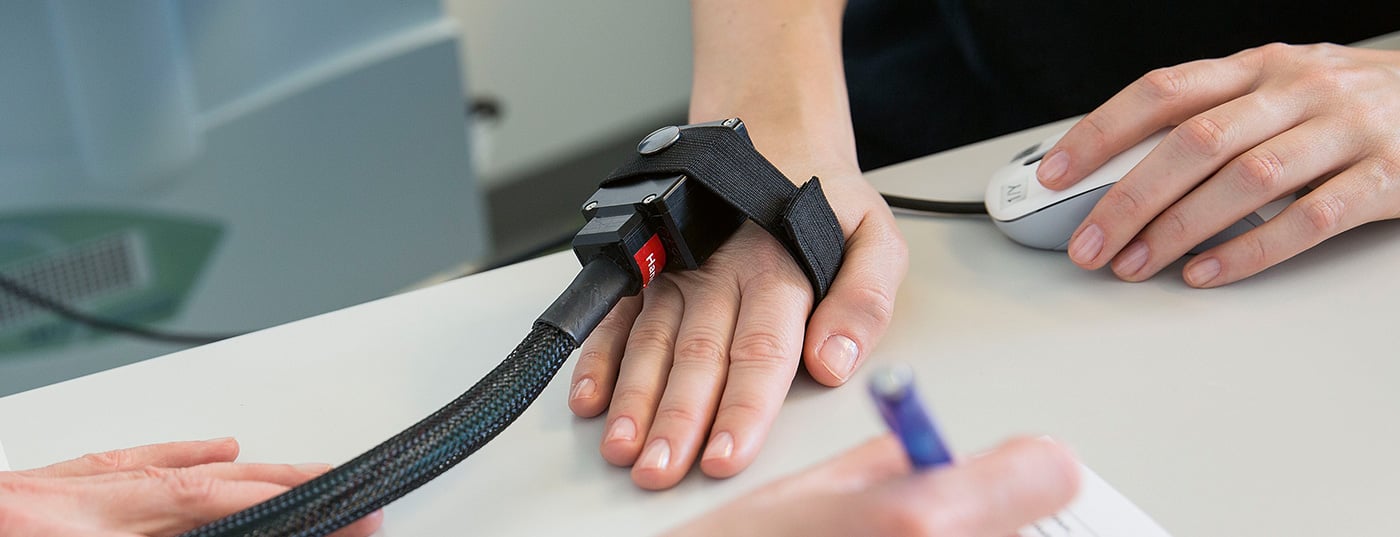
Thermal testing
Thermal testing examines the functionality of thin, low-myelination Aδ-fibers and non-myelination C-fibers and their pathway systems. It is performed by means of a computer-assisted thermometer (as an example Thermal Sensory Analyser II [TSA], Fig. 2). With the aid of a thermode (Peltier element), computer-controlled thermal stimuli are applied to the corresponding skin and mucous membrane area, starting with a base temperature of 32 °C and slowly increasing with stimulus intervals of 1 °C. The stimuli are then applied to the skin and mucous membrane area. Sensory thresholds for cold sensation (CDT), heat sensation (WDT), cold-induced pain (CPT), heat-induced pain (HPT), and thermal difference threshold (TSL) and whether cold stimuli are perceived as hot (paradoxical heat sensation, PHS) are determined [4].
Mechanical testing
Mechanical testing consists of several individual tests performed as follows.
Mechanical detection threshold (MDT): This is done with a set of standardized glass fiber filaments of different diameters and varying lengths (von Frey filaments) (Fig. 3A). The filaments are always placed in the same way until the filament bends in an s-shape to ensure accurate testing. This activates low-threshold mechanoreceptors that mediate the perception of touch via Aβ-fibers [4,14].
Mechanical pain threshold (MPT): Needle stimulators (PinPrick) are used for this purpose (Fig. 3B) . These are blunt needles with a fixed stimulation intensity and a circular skin contact surface. This predominantly activates the Aδ-nociceptors. The skin is not injured in the process [4].
Stimulus-response functions: The stimulus-response functions are used to determine the mechanical pain sensitivity (MPS) and to detect any dynamic mechanical allodynia (DMA). A set consisting of the previously mentioned needle stimulators, a cotton swab, a soft brush, and a cotton swab (Fig. 3C) is used [4].

Wind-up phenomenon (WUR, wind up ratio): The needle stimulators are used for this purpose. In the test area, the sensitivity of the skin to a single stimulus is compared with that to a series of stimuli. The wind-up quotient is calculated from the ratio of pain intensity (on the numerical analog scale: “0” no pain, “100” maximum imaginable pain) across the stimulus series divided by the pain intensity after the individual stimuli [4]. Wind-up represents a temporal summation phenomenon in the spinal cord that occurs specifically in wide-dynamic-range (WDR) neurons when their C-fiber inputs are stimulated more than once in 3 seconds [14]. The presence of a WUR is always pathologic and indicates a persistent increase in pain.
Vibration detection threshold (VDT): It is determined by means of a calibrated tuning fork (Rydel-Seiffer Vibration Fork®) as in the neurological examination (Fig. 3C). This is the only test within the QST battery where a “disappearance threshold” is determined [4,14]. It is mediated via Aβ-fibers.
Pressure pain threshold (PPT): Here, the transition of the perception of pressure quality into a painful sensory impression is determined [4]. A pressure algometer is used for this purpose (Fig. 3D).
Data evaluation
The collected QST parameters, before being transformed into a normal distribution, are logarithmically transformed, tabulated and graphically displayed. The values are compared with those of an age- and sex-specific normative collective for the assessment of pathological changes. Values outside the 95% confidence interval (± 1.96) are considered pathological [14]. The non-nociceptive parameters are CDT, WDT, TSL, MDT, VDT [15].
QST parameters
Rolke et al. were able to show in a study that the intraindividual comparison of the QST parameters of the right and left side of the body shows a high correlation and thus there is no difference between the two sides of the body in healthy individuals [16]. Age dependence and sex specificity of QST values have been shown in several studies. Among other things, there is an increase in perceptual and pain thresholds with age [16,17].
QST – Strength and Weakness
QST is a psychophysical procedure and therefore always dependent on the active cooperation of the patient. The experience of the examiner and the findings also play a crucial role. QST is not a substitute for clinical examination and no diagnosis can be made by QST alone. A strength of QST is that it is a non-invasive, side-effect-free examination. Compared to other methods for examining the function of small-caliber nerve fibers, QST can detect not only minus signs but also plus signs.
QST does not allow elevation localization or etiologic assignment of a lesion [4,18,19]. A QST finding may be normal despite a demonstrated reduction in the density of intraepidermal nerve fibers in the skin biopsy [18].
The data on diagnostic sensitivity and specificity in the diagnosis of small-fiber neuropathy are controversial [20]. Here, partial preference is given to more objective procedures such as morphometric examination of skin (skin biopsy to determine intraepidermal nerve fiber density) and corneal innervation (corneal confocal microscopy, among others, to determine corneal nerve fiber density). Both methods have their focus on the morphology of small caliber nerve fibers, so the methods complement each other. It should be kept in mind that a normal finding on both QST and skin biopsy does not rule out neuropathic pain and small-fiber affection any more than it proves either [19]. Results should always be assessed in the overall context of the history and clinical examination.
From symptom to mechanism
The pathophysiologic changes underlying the development of neuropathic pain usually occur independently of the etiology of the primary nerve injury. The pattern of QST profiles with loss of function and increase in function allows conclusions to be drawn about the underlying mechanisms. If a noxious stimulus is persistent, it leads to permanent excitation and thus to sensitization [21]. Different mechanisms are distinguished.
Deafferentation: evidence of sensory deafferentation is seen when there is damage to different types of fibers or their associated pathway systems in the spinal cord. This increases the expression of tetrodotoxin-sensitive sodium channels (e.g. NaV1.3) [22]. This is manifested in the QST profile by the presence of minus signs.
Peripheral sensitization: This is caused by the local release of inflammatory mediators (including histamine, bradykinin, prostaglandin, CGRP, substance P). Signal transduction and sensitization are mediated by glutamate, substance P, neurokinin A, CGRP, among others [9]. In the QST examination, peripheral sensitization is suspected by the presence of, for example, heat hyperalgesia [22].
Central sensitization: it results from an increased response of central WDR neurons. In QST, mechanical hyperalgesia to needle stimuli and dynamic mechanical allodynia are found [22].
Disruption of endogenous pain inhibition – central disinhibition: this exists when there is evidence of generalized hyperalgesia. This argues for a failure of pain inhibition (disruption of central pain modulators, periaqueductal gray (PAG)-RVM system (dorsal ventromedial medulla)), whereas localized hyperalgesia argues for central sensitization [23].
Possible applications for neuropathic pain
QST can be used in the diagnosis of neuropathic pain of any cause, especially when conventional electrophysiological methods show no abnormality and/or there is suspicion of affection of the small caliber nerve fibers or associated central pathways.
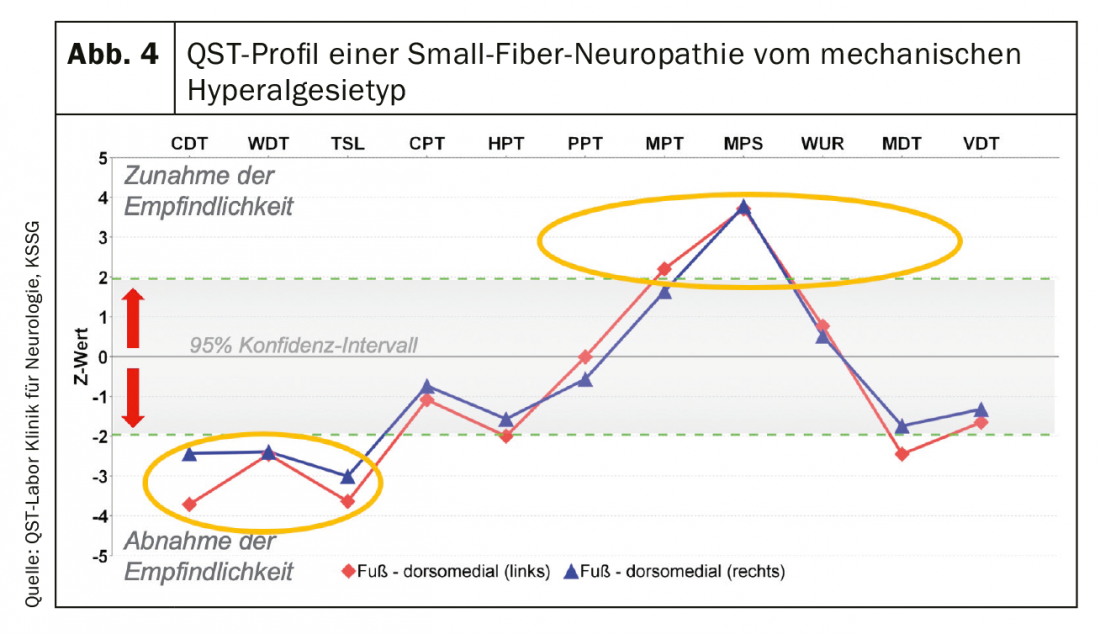
Small-fiber neuropathy (SFN): A gold standard in SFN diagnosis has not yet been defined. The diagnosis is made when at least two pathologic findings of the following examinations are present (clinical examination, functional testing, and/or small caliber nerve fiber morphology) [20,24]. Of all the special functional tests, QST is the most established procedure in the clinic. A typical finding is shown in Figure 4 .
Diabetic neuropathy (DPN): chronic symmetric length-dependent sensorimotor polyneuropathy is typical [19]. Approximately 25% of all diabetic patients develop painful DPN, which is associated with small caliber nerve fiber dysfunction rather than thick nerve fiber dysfunction [25,26].
QST is also used for facial pain (myofascial pain DD: trigeminal neuropathy/neuralgia, burning mouth syndrome) as well as in the oral region [27], in chemotherapy-induced polyneuropathies [28], postherpetic neuralgia [12], musculoskeletal pain [29] and as a postoperative course parameter [30]. Pattern profiles of the most common neuropathic pain syndromes were established as part of a multicenter study [15].
QST is also used in studies of fibromyalgia and small fiber pathology. It has been shown that QST findings and clinical neurological examination differ between patients with fibromyalgia syndrome (FMS) and patients with idiopathic SFN [31]. The “chicken and egg” question arises in fibromyalgia. It is unclear whether the mechanisms contribute to the pathophysiology of FMS or are consequences of FMS or its comorbidities. Research is needed to identify subgroups and develop subgroup-specific therapies.
Mechanisms-based therapy
The concept of mechanism-based therapy is based on the assumption that the different symptoms of neuropathic pain are due to different biological mechanisms and require a specific therapy that should be tailored exactly to the needs of the patient [8]. The pursued goal with the QST examination is to establish a pattern of the present pain profile, with the help of which conclusions can be drawn about the probable underlying pathophysiological mechanisms and thus statements can be made about the therapy concept of the respective patient [15]. This concept could not be implemented so far.
One of the most studied diseases to test the feasibility of the concept of mechanism-based therapy is diabetic neuropathy. The results raise more new questions than they clarify any. Raputova et al. have found that both QST profile and intraepidermal nerve fiber density are not predictive of the occurrence of pain in patients with diabetic polyneuropathy [32]. Meanwhile, Segerdahl et al show that QST parameters differ in patients with and without neuropathic pain [33]. Patients with painful diabetic neuropathy and genetically confirmed irritable nociceptors due to mutation in the sodium channel (NaV1.7) show less prominent sensory QST profiles than those without mutation, raising questions about the mechanistic significance of QST profiles [34]. The QST profile cannot be used to infer treatment success (e.g., response to oxcarbazapine for neuropathic pain in the setting of diabetic neuropathy) [35].
The hope of using QST profiles to move closer to the concept of mechanism-based therapy requires further clinical research. This is the basis for the prospect of being able to offer new treatment options in the future to patients who are often significantly and persistently impaired. It should not be ignored that the development of neuropathic pain is not only based on biological changes, but also has psychosocial aspects.
Take-Home Messages
- Quantitative sensory testing is a standardized and formalized noninvasive examination of the function of the entire somatosensory system, including small caliber nerve fibers.
- The testing allows the detection of sensitive plus and minus signs in contrast to conventional electrophysiology (e.g. neurography), which is limited to the functional deficit.
- QST is a psychophysical procedure that requires the patient’s cooperation.
Literature:
- Finnerup NB, et al: Neuropathic pain: an updated grading system for research and clinical practice. Pain 2016; 157(8): 1599-1606.
- Treede RD, et al: Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 2008; 70(18): 1630-1635.
- Schlereth T, et al: Diagnosis and noninterventional therapy of neuropathic pain, S2k guideline 2019; in: German Society of Neurology (ed.), Guidelines for Diagnosis and Therapy in Neurology.
- Rolke R, et al: Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006; 123: 231-243.
- Ahcan U, et al: Nerve fibre composition of the palmar cutaneous branch of the median nerve and clinical implications. Br J Plast Surg 2003 Dec; 56(8): 791-796.
- Hines AE, et al: Fiber type composition of articular branches of the tibial nerve at the knee joint in man. Anat Rec 1996 Dec; 246(4): 573-578.
- Klinke, Pape, Kurtz, Silbernagl: Physiology, Thieme, 2009.
- Baron R, et al: Pain and QST: “measure what is measurable”. Pain 2009 Feb; 23(1): 5-6; doi: 10.1007/s00482-009-0775-8.
- Julius D, et al: Molecular mechanisms of nociception. Nature 2001 Sep 13; 413(6852): 203-210.
- Magerl W, et al: Reference data for quantitative sensory testing (QST): refined stratification for age and a novel method for statistical comparison of group data. Pain 2010; 151: 598-605.
- Geber C, et al: Test-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): a multi-center study. Pain 2011 Mar; 152(3): 548-556.
- Pfau DB, et al: Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): reference data for the trunk and application in patients with chronic postherpetic neuralgia. Pain 2014 May; 155(5): 1002-1015; doi: 10.1016/j.pain.2014.02.004. epub 2014 Feb 10.
- Gröne E, et al: Test order of quantitative sensory testing facilitates mechanical hyperalgesia in healthy volunteers. J Pain 2012 Jan; 13(1): 73-80. doi: 10.1016
- Mücke M, et al: Quantitative sensory testing. Pain 2014 Dec; 28(6): 635-646.
- Maier C, et al: Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): somatosensory abnormalities in 1236 patients with different neuropathic pain syndromes. Pain 2010 Sep; 150(3): 439-450.
- Rolke R: Quantitative sensory testing: mechanism-based diagnosis of chronic pain syndromes. Habilitationsschrift, self-published in Mainz 2010.
- Dimova V, et al: Using a Standardized Clinical Quantitative Sensory Testing Battery to Judge the Clinical Relevance of Sensory Differences Between Adjacent Body Areas. Clin J Pain 2017 Jan; 33(1): 37-43.
- 18. Scherens A, et al: Painful or painless lower limb dysesthesias are highly predictive of peripheral neuropathy: comparison of different diagnostic modalities. Eur J Pain 2009 Aug; 13(7): 711-718; doi: 10.1016.
- Krumova EK, et al: Neuropathic pain: is quantitative sensory testing helpful? Curr Diab Rep 2012 Aug; 12(4): 393-402; doi: 10.1007/s11892-012-0282-7.
- Devigili G, et al: The diagnostic criteria for small fiber neuropathy: from symptoms to neuropathology. Brain 2008 Jul; 131(Pt 7): 1912-1925; doi: 10.1093.
- Mense SS, et al: Functional neuroanatomy for pain stimuli. Reception, transmission, and processing. Pain 2004 Jun; 18(3): 225-237.
- Treede RD et al: Hyperalgesia and Allodynia: taxonomy, assessment, and mechanisms. In: Brune K, Handwerker HO (eds): Hyperalgesia: molecular mechanisms and clinical implications. IASP Press, Seattle 2004; 991-1015.
- Gracely RH, et al: Evoked pain measures in fibromyalgia. Best Pract Res Clin Rheumatol 2003 Aug; 17(4): 593-609.
- Blackmore D, et al: Diagnostic Criteria for Small Fiber Neuropathy. J Clin Neuromuscul Dis 2017 Mar; 18(3): 125-131.
- Pfau DB, et al: Technical and clinical performance of the thermo-test device “Q-Sense” to assess small fiber function: A head-to-head comparison with the “Thermal Sensory Analyzer” TSA in diabetic patients and healthy volunteers. Eur J Pain 2019 Nov; 23(10): 1863-1878.
- Ekman L, et al: Evaluation of small nerve fiber dysfunction in type 2 diabetes. Acta Neurol Scand 2020 Jan; 141(1): 38-46.
- Hartmann A, et al: Profiling intraoral neuropathic disturbances following lingual nerve injury and in burning mouth syndrome. BMC Oral Health 2017 Mar 23; 17(1): 68.
- Roldan CJ, et al: Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers Modulate the Function of Myelinated Fibers after Chemotherapy: A Quantitative Sensory Testing Study. Pain Physician 2017 May; 20(4): 281-292.
- Georgopoulos V, et al: Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta-analysis. Pain 2019 Sep; 160(9): 1920-1932.
- Huber JL, et al: Recovery of mechanical detection thresholds after direct digital nerve repair versus conduit implantation. J Hand Surg Eur 2017 Sep; 42(7): 720-730.
- Üceyler N, et al: Small fiber pathology in patients with fibromyalgia syndrome. Brain 2013 Jun; 136(Pt 6): 1857-1867.
- Raputova J, et al: Sensory phenotype and risk factors for painful diabetic neuropathy: a cross-sectional observational study. Pain 2017 Dec; 158(12): 2340-2353.
- Segerdahl AR, et al: A brain-based pain facilitation mechanism contributes to painful diabetic polyneuropathy. Brain 2018 Feb 1; 141(2): 357-364.
- Blesneac I, et al: Rare NaV1.7 variants associated with painful diabetic peripheral neuropathy. Pain 2018 Mar; 159(3): 469-480.
- Gierthmühlen J, et al.: Can self-reported pain characteristics and bedside test be used for the assessment of pain mechanisms? An analysis of results of neuropathic pain questionnaires and quantitative sensory testing. Pain 2019 Sep; 160(9): 2093-2104.
InFo PAIN & GERIATry 2020; 2(1): 6-11.