The most common water balance disorder is hyponatremia; thus, it is also the most common electrolyte disorder. The symptoms are varied from mild to life-threatening. The severity of symptoms depends on the rapidity of development, duration, and severity of hyponatremia. The clarification, but also the therapy of this electrolyte disorder is not always easy.
Hyponatremia is usually not due to a sodium deficiency, but primarily to a disturbance in the body’s fluid balance. From the point of view of balance, the pathogenesis of hypotensive hyponatremia is remarkably simple: either too much hypotensive fluid is ingested or too little of it is excreted in the urine, explains Dr. Cédric Jäger, Senior Physician Nephrology, University Center for Internal Medicine at the Cantonal Hospital Baselland, Bruderholz site [1]. To excrete relevant amounts of hypotonic urine, the kidneys must dilute the urine by reabsorbing more sodium and potassium than water, maintain this dilution, and excrete sufficiently large amounts of nonelectrolyte solutes, generally urea. Accordingly, for clinical purposes, four basic mechanisms of hyponatremia can be distinguished, Jäger added: high free water intake, high antidiuretic hormone (ADH), dilutional defect, and low excretion of dissolved nonelectrolytes.
Two central parameters decisive
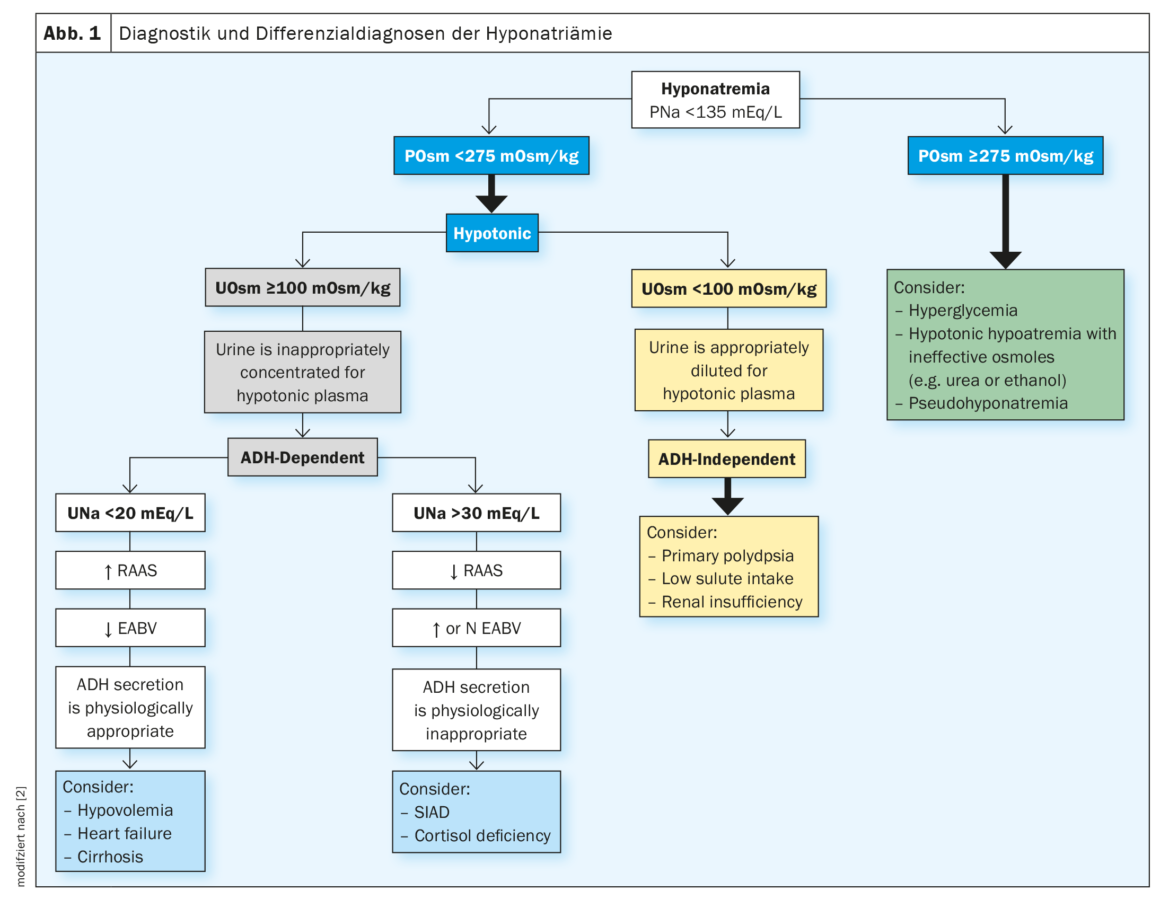
Hyponatremia is defined as a serum sodium level <135 mmol/l. When hyponatremia occurs, plasma osmolality is first determined. A plasma osmolality <275 mOsm/kg indicates hypoosmolar hyponatremia. In this case, urine osmolality should be determined. At a urine osmolality <100 mOsm/kg, ADH is not active, urine is adequately diluted for hypotonic plasma, primary polydipsia, low solute intake, or renal insufficiency may be considered. A urine osmolality ≥100 mOsm/kg indicates active ADH, urine becomes inappropriately concentrated for hypotonic plasma. If this is the case, urine sodium (UNa) should be determined. At a UNa <20 mEq/l, the renin-angiotensin-aldosterone system (RAAS) is active and effective arterial blood volume (EABV) is low. ADH secretion is physiologically adequate; hypovolemia, heart failure, or cirrhosis may be considered. At a UNa >30 mEq/l, RAAS is not active and effective arterial blood volume (EABV) is increased. ADH secretion is physiologically inappropriate. The syndrome of inadequate antidiuresis (SIADH) develops, whereby the body retains fluid and lowers sodium levels by dilution (Fig. 1) [2].
Low solute intake is an underestimated cause of hyponatremia
Most patients with hyponatremia excrete hypotonic urine, with the exception of SIADH. In this case, the excretion rate of free water depends on the urine volume, which in turn depends on the excretion rate of solutes. The daily amount of solutes to be excreted in adults on a normal diet is 500-1000 mOsm and consists of urea, produced by the metabolism of dietary protein, and electrolytes. If a patient has a daily dissolved load of 600 mOsm, he will excrete 3 liters of urine per day (600 mOsm/200 mOsm/kg) and thus 1.5 liters of electrolyte-free water per day. The likelihood of this patient becoming hyponatremic with a normal daily water intake of 1.5 liters is very low. On the other hand, if the daily dissolved charge drops to 300 mOsm, for example, due to inadequate protein intake, the daily excretion of electrolyte-free water would be only 750 ml. In this situation, limiting water intake to about 750 ml daily would be necessary to prevent progressive hyponatremia. Finally, a patient with a daily dissolved load of 150 mOsm would have a daily electrolyte-free water excretion of 375 ml and would likely become progressively hyponatremic despite severe water intake restriction.
The many faces of a common clinical picture
The extreme manifestation of this phenomenon is called “beer potomania”. It occurs rarely and mainly in alcoholics who drink large amounts of low-electrolyte fluid and consume little protein and develop hyponatremia despite normal urine diluting ability. This has also been described in patients who follow extreme weight loss diets with very low protein and high water intake (e.g., a “tea and toast” diet), which is referred to as “non-beer potomania” or “starvation potomania.”
Also underestimated is that low solute intake is a common contributing factor to hyponatremia in patients with other conditions that cause hyponatremia. In particular, patients with chronic heart failure and chronic liver failure are usually highly salt-restricted and may have a restricted appetite. In addition, many of these patients are elderly, live alone, and are severely dysfunctional, further limiting their ability and motivation to prepare nutritious meals. These patients have a urinary dilution defect because the circulating volume is depleted and the secretion of ADH is stimulated. It has been noted that many of these patients have a relatively mild urinary dilution defect but have disproportionately severe hyponatremia that is unexpectedly resistant to the primary treatment of fluid restriction. “Potomania” is a misnomer in this context, as sufferers often consume very little fluid either voluntarily or because of prescribed fluid restriction.
Pay attention to protein intake in the diet
This presents a difficult management problem because there are few other therapeutic options in such patients: Saline tablets are usually contraindicated because of edema, tolvaptan is approved only for acute treatment up to one month, and is contraindicated in liver disease. Loop diuretics are effective in lowering very high urine osmolality, but generally only to 200-300 mOsm/kg and not below. In such patients, anecdotal success has been achieved with treatment by increasing dietary protein intake. In this way, urea formation, daily osmolar load and thus daily free water excretion can increase. Interestingly, this is physiologically equivalent to treating patients with urea, which has been used successfully to treat SIADH.
It is recommended that the daily solute excretion rate be determined in all patients with hyponatremia. This can be determined using a 24-hour urine collection: Osmolar excretion rate (mOsm/day) = urine osmolality (mOsm/kg) × urine volume (l/day). Alternatively, osmolality can be estimated from a puncture urine by normalizing urine osmolality to creatinine concentration: Osmolar excretion rate (mOsm/day) = urine osmolality (mOsm/kg)/urine CR concentration (mg/dl) × 100. If the osmolar excretion rate is <500 mOsm/kg, the patient’s protein intake should be estimated. This can be done either directly by evaluating self-reported dietary intake or indirectly from measured urinary urea levels using an estimated protein nitrogen occurrence equation. If the patient’s protein intake is low, measures should be initiated to increase protein intake to increase free water excretion and improve hyponatremia.
Congress: FomF Update Refresher 2023
Literature:
- Dr. med. Cédric Jäger: Elektrolytstörungen – Richtig diagnostizieren und therapieren. Forum Medizin Fortbildung (FomF), Update Refresher 2023, Nephrologie, 26.01.2023.
- Workeneh BT, et al.: Hyponatremia Demystified: Integrating Physiology to Shape Clinical Practice. Advances in Kidney Disease and Health, 2022;
doi: https://doi.org/10.1053/j.akdh.2022.11.004.
HAUSARZT PRAXIS 2023; 18(2): 32-33 (published 2/22/2013, ahead of print).











