Hematopoietic stem cell transplantation (HSCT) is used as ultima ratio in multiple sclerosis. The reboot of the immune system is aimed at healing, but carries strong side effects. Both infectious and noninfectious pulmonary complications can lead to increased morbidity and mortality. Most recently, success has been achieved in the prophylaxis and treatment of infectious complications, which brings the importance of noninfectious lung diseases to the forefront.
Pulmonary complications occur in up to one-third of HSCT patients. Factors associated with increased risk of lung complications include age, graft-versus-host disease (GvHD), stem cell source, and underlying lung disease, writes a team led by Samran Haider, M.D., of the Division of Pulmonary, Critical Care and Sleep Medicine at Wayne State University School of Medicine, Detroit [1]. To identify patients at high risk for developing pulmonary complications, respiratory failure, and/or mortality after HSCT, pre-transplant pulmonary function tests (PFT) – including forced expiratory volume in 1s (FEV1) – and diffusing capacity of the lung for carbon monoxide (DLCO) are available. Pre-transplant smoking may also be an independent predictor of long-term complications and death. Antimicrobial prophylaxis and treatment strategies have been effective in reducing the incidence of infectious lung complications after HSCT, but the incidence of noninfectious lung injury continues to rise. Also, improvement in supportive measures has led to better survival after acute noninfectious pulmonary complications, thus increasing the importance of late noninfectious complications (such as bronchiolitis-obliterans syndrome, BOS, and interstitial lung disease, ILD).
Diagnosis of non-infectious pulmonary complications
For the management of pulmonary complications after HSCT, it is important that all patients are evaluated by thorough history, physical examination, PFT, and chest radiography before transplantation. Chest CT scans may be indicated especially in elderly patients, smokers, or patients with an abnormal initial evaluation. These studies should serve as a basis for post-transplantation changes.
Dr. Haider advises that respiratory symptoms in the early posttransplant period (generally the first 100 days) should be evaluated in the context of symptom severity and the patient’s immune status (neutrophil count, immunosuppressive medications, presence of acute GvHD, and antimicrobial prophylactic measures). Infections should be considered first during this time. High-resolution chest CT can provide information on the etiology of the patient’s symptoms. Bronchoscopy with bronchoalveolar lavage (BAL) is well tolerated and leads to a diagnosis in about half of patients. Surgical lung biopsies are now rarely required after HSCT, and the decision to proceed with this procedure should be made in a multidisciplinary approach and on a case-by-case basis.
In the late post-HSZT period, chronic noninfectious pulmonary complications, including BOS, ILD, or mixed changes, become more important. Once the damage associated with these conditions has been identified, treatment options are limited. Dr. Haider et al. therefore recommend that patients be carefully monitored after HSCT by regular outpatient visits and review of respiratory symptoms. Screening spirometry should be performed every 3 months after the first 100 days and for the first 2 years. The presence of a new obstructive pattern compared to baseline is suggestive of bronchiolitis-obliterans syndrome, whereas a new restrictive finding is suggestive of ILD. Also, the combination of new obstructive and restrictive changes may occasionally occur, reflecting mixed patterns of BOS and ILD. When changes occur and persist in PFT, HRCT is helpful in delineating lung disease. The findings of inhomogeneous air trapping on expiratory CT (mosaic pattern), small airway thickening, or bronchiectasis are consistent with BOS, whereas ILD associated with GvHD usually manifests radiologically with persistent multilobar opacities with or without pleural changes.
In a review, the authors summarized acute and chronic noninfectious pulmonary complications after HSCT, highlighting diagnostic criteria, incidence, pathogenesis, outcomes, and recent advances in management.
Idiopathic pneumonia syndrome (IPS)
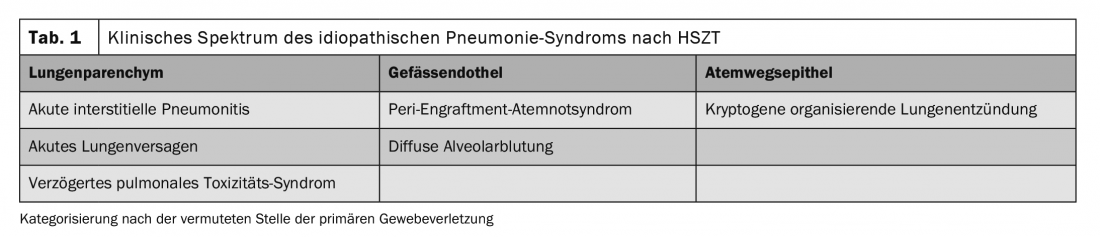
The American Thoracic Society defines idiopathic pneumonia syndrome (IPS) as an idiopathic pneumopathy following HSCT. The diagnosis of IPS requires evidence of widespread alveolar injury without concurrent infection, iatrogenic fluid overload, cardiac or renal failure. IPS occurs in both allogeneic and autologous HSCT patients and is further classified based on the presumed site of tissue injury (Table 1). The incidence of IPS after myeloablative preparative regimen is approximately 3-15%. Risk factors for IPS after allogeneic HSCT include conditioning at full intensity, total body irradiation, GvHD, age >40 years, and underlying diagnosis of acute leukemia or myelodysplastic syndrome. Bronchoscopy with bronchoalveolar lavage of the affected areas is important to rule out an infectious process.
Peri-engraftment respiratory distress syndrome (PERDS).
Peri-engraftment respiratory distress syndrome (PERDS) is a form of acute lung injury that occurs in a subset of patients with engraftment syndrome (ES). It is defined as hypoxemic respiratory failure and bilateral pulmonary infiltrates occurring at the time of transplantation that cannot be fully explained by cardiac dysfunction or infection. PERDS is reported less frequently with allogeneic HSCT than with autologous.
Although the exact mechanisms remain unclear, it is postulated that the role of activated granulocytes releasing proinflammatory cytokines such as interleukin(IL)-1β, IL-2, or IL-6 and the influx of neutrophils into the lung during transplantation play a primary role. In the allogeneic setting, it can be difficult to distinguish PERDS from acute GvHD because of significant overlap in clinical symptoms. It appears that less damaged stem cells, endothelial cells, and tissues release more proinflammatory cytokines at the time of transplantation, facilitating the development of this syndrome.
Clinical clues to the diagnosis include systemic inflammatory manifestations such as diffuse rash, diarrhea, hepatic dysfunction, renal dysfunction, transient encephalopathy, and other capillary leak features such as noncardiogenic pulmonary infiltrates, hypoxia, and weight gain with no alternative etiologic basis other than transplantation. The recommended treatment for PERDS includes immediate treatment with a high dose of corticosteroids (1 to 2 mg/kg-1 methylprednisolone twice daily for 3 days), followed by rapid reduction. Response is typically rapid, with oxygenation improving in most patients within a few days of starting treatment. Supportive measures include antipyretics, oxygen, diuretics, and intubation/mechanical ventilation.
Diffuse alveolar hemorrhage (DAH)
Diffuse alveolar hemorrhage (DAH) is an IPS subtype defined as BAL and may involve various manifestations such as dyspnea, nonproductive cough or hemoptysis, and hypoxemia with or without fever. Also observed, among others, is an increasingly bloody return fluid in serial lavages, ≥20% hemosiderin-loaded macrophages, or blood in at least 30% of alveolar surfaces. DAH is characterized by rapid progression of respiratory failure and is considered a sign of an underlying lung injury influenced by multiple risk factors rather than a disease in its own right.
Therapy remains empiric and therefore inadequate due to the unknown pathogenesis of the disease. Systemic corticosteroids are frequently used, but with unsatisfactory results. Supportive measures may also include platelet transfusions, procoagulant therapies (aminocaproic acid and recombinant factor VIIa), and cytokine antagonists (etanercept, cyclophosphamide), which have been used in small studies with variable success.
In most cases, mechanical ventilation is required. Extracorporeal membrane oxygenation (ECMO) has been used as a rescue therapy in the treatment of severe lung injury associated with DAH and other forms of IPS. However, because of poor survival rates, use should be evaluated on an individual patient basis.
Cryptogenic organizing pneumonia (COP).
Cryptogenic organizing pneumonia (COP) was formerly known as bronchiolitis obliterans. It is a syndrome consisting of nonspecific respiratory symptoms (fever, dyspnea, and cough), patchy consolidation on imaging, and a restrictive ventilatory defect on pulmonary function tests. COP is more common after allogeneic HSCT, where it has an incidence between 1 and 10%. It usually occurs between 2 and 15 months after transplantation.
Risk factors include female-to-male HSCT, HLA incompatibility, acute or chronic GvHD, and peripheral blood stem cell transplantation. Symptoms are nonspecific and include fever, shortness of breath, and cough. COP is frequently associated with GvHD of the skin. Pulmonary function tests have shown that restrictive ventilatory defect, FEV1, forced vital capacity, total lung capacity, and DLCO are significantly reduced.
COP is treated with corticosteroids over a prolonged period of time. Patients are typically treated with a prednisone dose of 0.5-1 mg/kg-1 with slow reduction. Relapses are common and may occur when steroids are reduced.
Bronchiolitis-obliterans syndrome (BOS)
BOS is characterized by new-onset airflow limitation after allogeneic HSCT. It is also reported in patients with inhalation exposure, rheumatoid arthritis, and patients who have undergone lung transplantation. Chronic GvHD (especially skin and eye) is known to be associated with BOS. Symptoms may include dyspnea on exertion, coughing, or wheezing, although many patients are asymptomatic early in the disease process. Diagnosis of BOS requires a PFT and expiratory CT chest.
Disease manifestations usually occur after approximately 100 days and within the first 2 years after allogeneic HSCT. The clinical course is variable, with some patients showing rapid decline in pulmonary function and others showing slowly progressive disease with episodes of exacerbation. PFT screening 100 days and 1 year after transplantation or at initial diagnosis of chronic GvHD is recommended, as is further PFT screening at 3 month intervals for the first 2 years after initial diagnosis of chronic GvHD.
Treating BOS is a challenge. Corticosteroids are not recommended due to side effects. Retrospective observational studies have shown an improvement in clinical status and an increase in FEV1 in BOS patients treated with azithromycin. Montelukast was recently studied in patients who developed BOS after lung transplantation and showed a slowing of FEV1 decline at 1 year compared with placebo in stage 1 after lung transplantation. One study investigated the use of inhaled budesonide/formoterol in patients with BOS after allogeneic HSCT. The study showed a median increase in FEV1 of 240 ml. The increase was maintained at the 6-month follow-up. However, despite the improvement in FEV1, patients did not report improvement in respiratory symptoms.
Interventions supporting the management of BOS after HSCT include early detection and treatment of respiratory tract infections, treatment of gastroesophageal reflux disease, and pulmonary rehabilitation.
Increasing importance
Non-infectious pulmonary complications are becoming increasingly important in patients after HSCT. The diagnostic criteria and terminology for these disorders remain confusing because of the significant overlap between clinical entities and their coexistence with infectious complications, Dr. Haider and colleagues summarize. As the number of HSCTs performed increases, knowledge of pulmonary complications following such a procedure becomes increasingly important. Unfortunately, well-designed clinical trials for the treatment of these diseases are lacking, the authors complain. A multicenter collaboration to collect data on risk factors, diagnostic approaches, and management strategies is therefore needed, he said.
Source:
- Haider S, et al: Eur Respir Rev 2020; 29: 190119; doi: 10.1183/16000617.0119-2019.
InFo NEUROLOGY & PSYCHIATRY 2021; 19(1): 29-31.