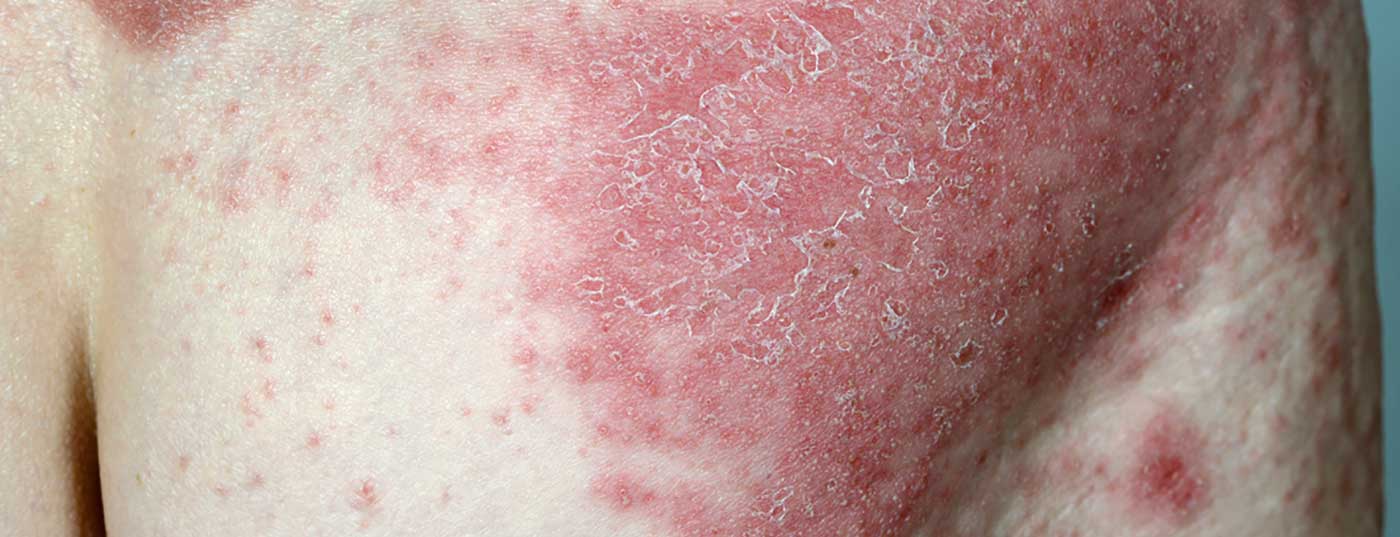
A long-term extension analysis of the ERASURE and FIXTURE studies recently published in the British Journal of Dermatology demonstrates the long-lasting efficacy and safety of secukinumab in patients with moderate-to-severe psoriasis. These data confirm the results of the long-term extension of the SCULPTURE study.
At ERASURE (Efficacy of Response And Safety of two fixed secUkinumab REgimens in psoriasis). and FIXTURE (Full year Investigative eXamination of secukinumab vs. eTanercept Using 2 dosing Regimens to determine Efficacy in psoriasis) These are two Phase III studies that demonstrated the efficacy and safety of secukinumab in moderate to severe psoriasis. [1,2]. In these two pivotal randomized, double-blind studies, different doses of secukinumab (Cosentyx®) were compared with placebo and etanercept [1–3]. Results were consistently in favor of secukinumab; the anti-IL17A antibody proved superior in terms of both PASI75** response rates and IGA0/1# [1,2].
** PASI75=reduction in the Psoriasis Area and Severity Index by ≥75% since baseline.
# IGA0/1 = score of 0 (lesion-free) or 1 (almost lesion-free) on a 5-point scale of the “modified Investigator’s Global Assessment”.
Long-term extension of ERASURE and FIXTURE
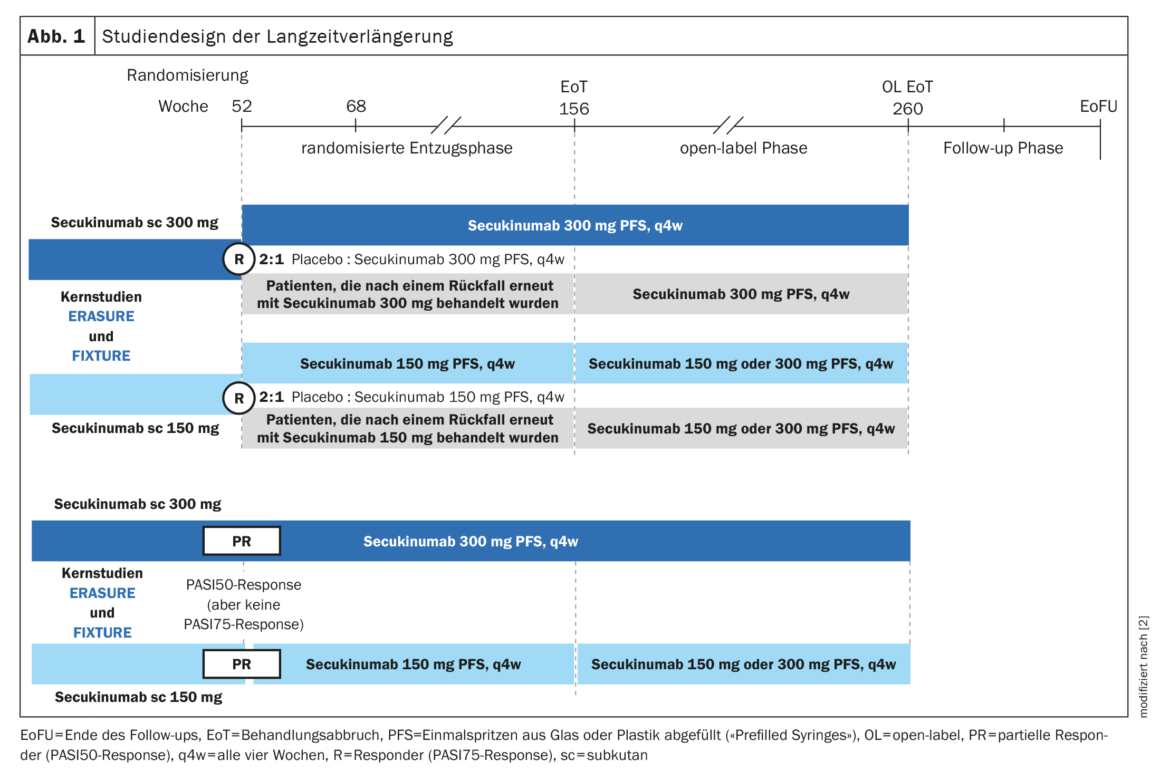
At the end of the core studies of the two pivotal phase III trials (i.e., after year 1), PASI75 responders (R) were randomly assigned in a 2:1 ratio to either the same dose of secukinumab (300 mg or 150 mg, continuous treatment) or placebo (discontinuation of treatment) every four weeks until year 3 [2]. Patients with partial response (PR), i.e. patients who had not achieved a PASI75 response but had achieved a PASI50 response, continued to receive the same dose of secukinumab that they had received during the core studies. Patients who relapsed on placebo received the same dose of secukinumab as before discontinuation. The total duration of blinded treatment discontinuation in the study was 2 years (ie, until year 3). In the third year, all patients received open-label treatment with secukinumab. For those who received the 300 mg dose, this dosage was maintained and those who received 150 mg of secukinumab were adjusted to 150 mg or 300 mg of secukinumab. The overall design of the extension study is shown in Figure 1 .
The most important results at a glance
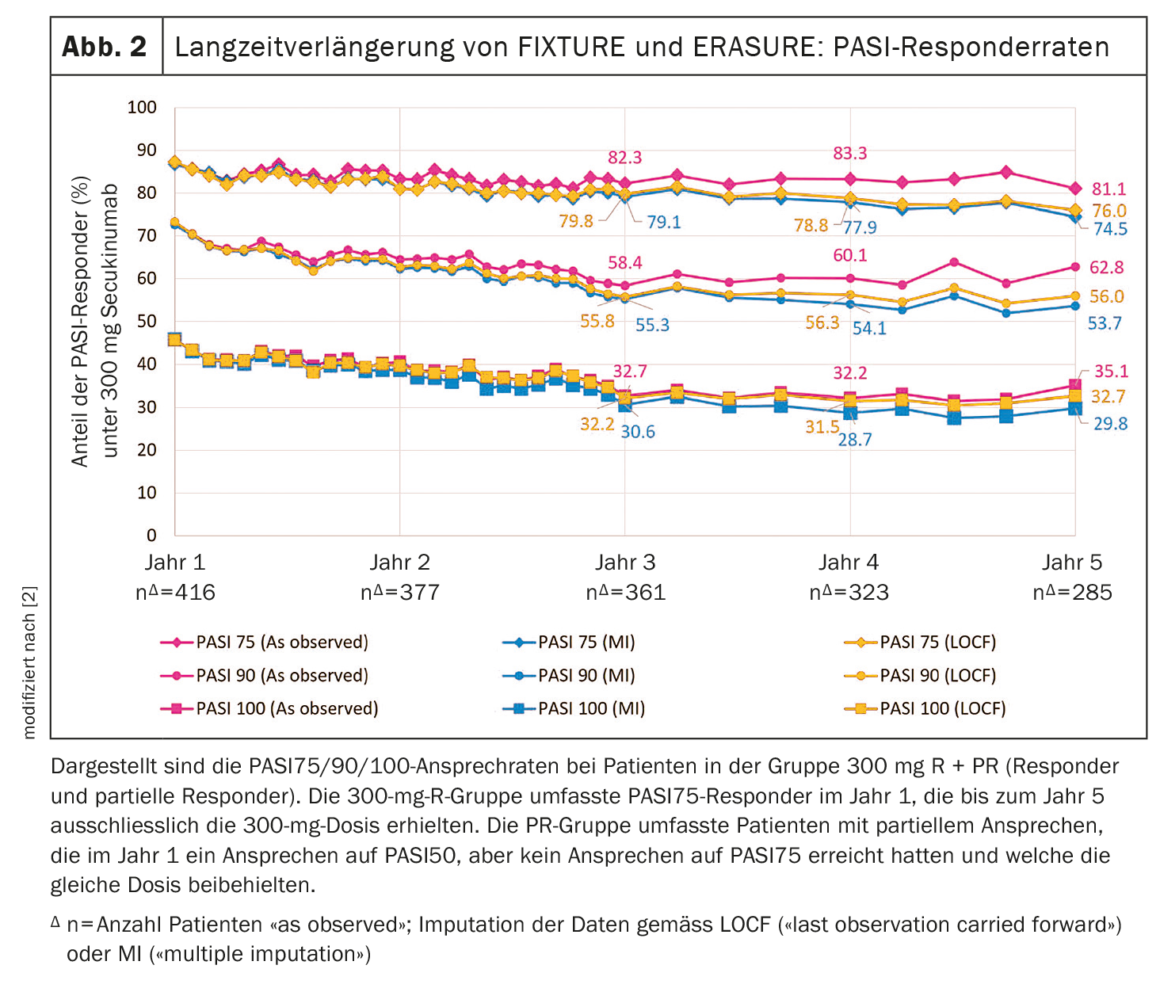
In the 300 mg R+PR group, PASI response was maintained with secukinumab until the fifth year of the extension study (PASI 75/90/
100, “as observed”: 81.1%/62.8%/35.1%) [2] (Fig. 2). PASI response for the 300 mg R + PR group trended downward after year 1 to year 3: from 86.8% to 82.3% and from 72.8% to 58.4%, respectively, and from 45.9% to 32.7% for PASI 75, PASI 90, and PASI 100. During years 4 and 5, PASI response rates stabilized (Fig. 2) . At all time points in the extension study, the PASI75 response (“as observed”) was ≥80%, and the PASI90 and PASI100 responses remained at 60% and 30%, respectively. This was the case even when different statistical approaches were used to impute missing data$, i.e., “multiple imputation” (MI) or “last observation carried forward” (LOCF). The PASI response rates of the long-term extension of ERASURE and FIXTURE are consistent with the results reported in the 4-year long-term extension of the SCULPTURE trial [4]. Also with regard to quality of life (“Dermatology Life Quality Index”, DLQI), the long-term data of ERASURE and FIXTURE show a continuous progression. In the R + PR group, 72.6%, 67.4%, 62.2%, 63.1%, and 60.8% of patients achieved a DLQI score of 0 or 1 (ie, little or no impairment in quality of life) at years 1, 2, 3, 4, and 5 of the study, respectively.
$ “Last Observation Carried Forward” (LOCF) and “Multiple imputation” (MI) are imputation procedures used in longitudinal analyses to deal with missing data. LOCF means that in case of missing values, the corresponding data value from the previous measurement is used. MI means that the missing values are replaced by multiple imputation, resulting in an unbiased estimate of the standard errors.
The safety profile with secukinumab was consistent throughout the observation period, with no increase in the frequency of adverse events over time. The incidence of treatment-emergent serious adverse events was low: 6.08 events per 100 patient-years at any secukinumab dose (n=198) and 5.01 events per 100 patient-years at the 300 mg dose (n=143).
Literature:
- Langley RG, et al: Secukinumab in plaque psoriasis-results of two phase 3 trials. N Engl J Med 2014; 371: 326-338.
- Langley RG, et al: Secukinumab long-term efficacy and safety in psoriasis through to year 5 of treatment: results of a randomized extension of the phase III ERASURE and FIXTURE trials. Br J Dermatol 2023; 188(2): 198-207.
- Drug Information, www.swissmedicinfo.ch,(last accessed Mar. 29 , 2023).
- Bissonnette R, et al: Secukinumab demonstrates high sustained efficacy and a favorable safety profile in patients with moderate-to-severe psoriasis through 5 years of treatment (SCULPTURE Extension Study).
J Eur Acad Dermatol Venereol 2018; 32: 1507-1514.
DERMATOLOGIE PRAXIS 2023; 33(2): 34-35