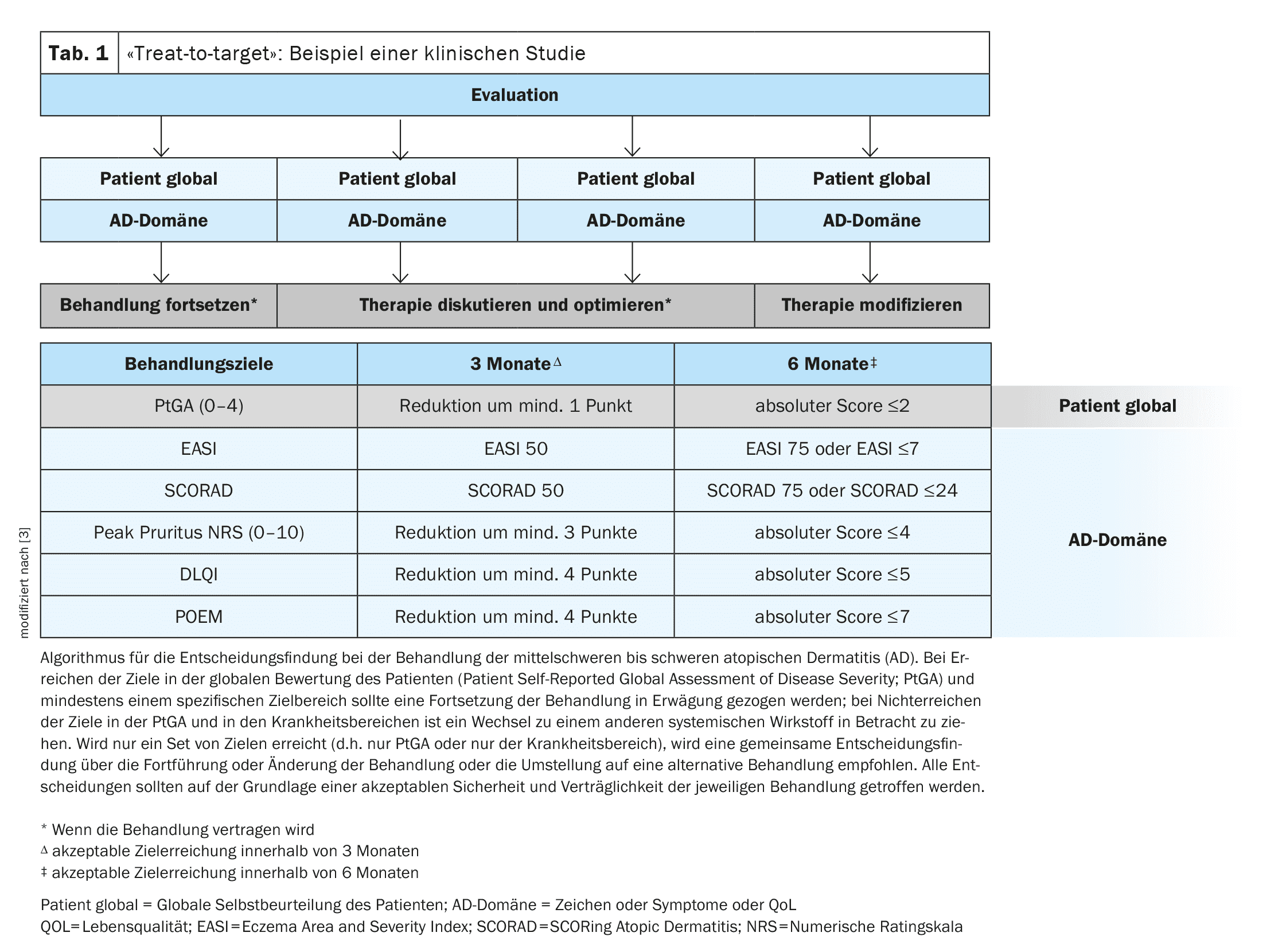
In the recent past, several secondary analyses have been published in which different treatment regimes for atopic dermatitis (AD) were compared and evaluated. In order to evaluate treatment progress using validated measurement instruments, more recent clinical studies are based on the HOME initiative, as Prof. Spuls, UMC Amsterdam, pointed out.
Defining and evaluating treatment goals is an important basic principle for achieving the best possible care for patients with atopic dermatitis (AD), emphasized Professor Phyllis I. Spuls, dermatologist at the University Medical Center (UMC) in Amsterdam (NL) [1]. The “Harmonizing Outcome Measures for Eczema” (HOME) initiative summarizes the recommended outcome parameters with the aim of standardizing the evaluation of treatment progress [2]. In a study by de Bruin-Weller et al., a “treat-to-target” approach was implemented, which has parallels to the approach established for psoriasis (Table 1) [3]. The following measurement instruments were used to assess AD-relevant parameters at baseline and during the course of the disease: EASI (Eczema Area and Severity Index), SCORAD (Scoring Atopic Dermatitis), numerical rating scale for pruritus (Peak Pruritus-NRS), DLQI (Dermatology Life Quality Index) and POEM (Patient-Oriented Eczema Measure) [3–6]. For each parameter, follow-up data was collected three and six months after baseline – these intervals correspond to suitable time periods for assessing the response to therapy [3]. These and other clinical studies have been incorporated into several subsequent meta-analyses and systematic reviews, which the speaker summarized [1].
Patient education: findings of a new Cochrane review
The fact that patient education is an important factor for successful AD therapy is also emphasized in current guidelines [7,8]. A Cochrane review published in 2024 with data from 6170 participants from 37 studies showed that supplementary patient education at the individual level leads to better short-term outcomes compared to standard therapy alone, while patient education in a group setting has a positive effect on AD symptoms in both the short and long term [9]. In addition to information on the multifactorial pathogenesis of AD and trigger factors such as irritants or stress, patients should be educated about basic measures: short daily showers/baths at a water temperature of 27-30°C are recommended to remove skin-irritating particles and bacteria. Ideally, emollients should be applied immediately afterwards (“soak and seal”) to prevent flares and strengthen the skin barrier. Explanations on topical anti-inflammatory topicals are also important, as these are an important pillar in the treatment of almost all AD patients.
Topical therapy: TCS and TCI currently still “frontrunners”
In Europe, topical corticosteroids (TCS) and topical calcineurin inhibitors (TCI) are currently mainly used for AD, while in the USA the Janus kinase (JAK) inhibitor ruxolitinib and the PDE inhibitors crisaborole and roflumilast are also approved in topical form [10]. In the EU, topical crisaborole has also been approved in the AD indication and roflumilast is expected to be approved soon[11]. A network meta-analysis (NMA) published in 2023 compared various topical AD therapies; data from a total of 43,123 pediatric and adult study participants were included [12]. Another NMA was published this year and included a total of 45,846 participants from 291 studies [13]. The majority were studies in adults, but 31 of the studies focused on <12-year-old children. The indirect comparison of the different topical treatment regimens was in favor of potent TCS, JAK inhibitors and tacrolimus 0.1%. Weak TCS, PDE-4 inhibitors and tapinarof 1% proved to be comparatively less effective. Furthermore, it was shown that no thinning of the skin was observed with short-term use of TCS, whereas this tended to be the case with longer-term use.
Light therapy: still in demand
A survey of 229 dermatologists in 30 European countries revealed that over 80% of respondents use light therapy in the treatment of AD [14]. Narrowband UVB phototherapy (NB-UVB) with a wavelength of 311-313 nm was used most frequently, according to the speaker [1,14]. The effect factors of NB-UVB in AD are described as follows:
- Immunomodulating effects: Suppression of cellular immunity; activation of innate immunity
- Strengthening the stratum corneum: permeability to pathogens is reduced
- Antipruritic effect: induction of apoptosis; inhibition of Langerhans cells and modification of cytokine production.
Prof. Spuls referred to a Cochrane Review published in 2021, which showed that NB-UVB was superior to placebo in terms of IGA, EASI and SCORAD after a 12-week period [15]. Various international efforts are underway to expand the evidence base on light therapy for AD, according to the speaker [1,16].
System therapy: registry data to facilitate therapy selection
A large arsenal of different systemic treapeutics is now available. The aim is to weigh up the benefits and risks on an individual basis. Comorbidities, vaccination status and the patient’s life situation should be taken into account. Overall, biologics have a favorable safety profile, especially as conjunctivitis, which occasionally occurs as a side effect, can be treated well [17]. Compared to biologics, oral JAK inhibitors require more preliminary clarification and monitoring measures. Although only ciclosporin is officially approved as a conventional systemic therapeutic agent, methotrexate (MTX), azathioprine and mycophenolate mefotil are not so rarely used in everyday clinical practice, according to the speaker [1]. In an NMA published in 2024, various system therapeutics are subjected to an indirect comparison [18]. A systematic review of MTX in AD was also published this year [19]. Last but not least, the speaker referred to the international TREAT registry study [20]. This is primarily concerned with evaluating the efficacy and safety of systemic therapy for AD in a long-term perspective in a real-world setting [1,21].
Congress: EADV Annual Meeting
Literature:
- “Hard to identify and treat AD”, Prof. Phyllis I. Spuls, Presentation ID D2T02.2C, EADV Annual Meeting, Amsterdam, 25-28.09.2024.
- Harmonizing Outcome Measures for Eczema (HOME), www.homeforeczema.org, (last accessed 26.22.2024).
- De Bruin-Weller M, et al: Treat-to-Target in Atopic Dermatitis: An International Consensus on a Set of Core Decision Points for Systemic Therapies. Acta Derm Venereol. 2021 Feb 17;101(2): adv00402.
- Schmitt J, et al: The Harmonizing Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. J Allergy Clin Immunol 2014; 134(4): 800-807.
- Légaré S, et al: Sensitivity of clinician-assessed efficacy outcome measurement instruments in trials of topical therapies for atopic dermatitis: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 2022; 36(2): 196-212.
- Silverberg JI, et al: Validation of five patient-reported outcomes for atopic dermatitis severity in adults. Br J Dermatol. 2020;182(1): 104-143.
- Wollenberg A, et al: European guideline (EuroGuiDerm) on atopic eczema: part I – systemic therapy. J Eur Acad Dermatol Venereol 2022; 36(9): 1409-1431.
- Wollenberg A, et al: European guideline (EuroGuiDerm) on atopic eczema – part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol 2022; 36(11): 1904-1926.
- Singleton H, et al: Educational and psychological interventions for managing atopic dermatitis (eczema). Cochrane Database Syst Rev. 2024 Aug 12;8(8):CD014932
- PharmaWiki, www.pharmawiki.ch,(last accessed 27.11.2024).
- Müller S, Maintz L, Bieber T: Treatment of atopic dermatitis: Recently approved drugs and advanced clinical development programs. Allergy 2024; 79(6): 1501-1515.
- Chu AWL, et al: Systemic treatments for atopic dermatitis (eczema): Systematic review and network meta-analysis of randomized trials. J Allergy Clin Immunol. 2023 Dec; 152(6): 1470-1492.
- Lax SJ, et al: Topical anti-inflammatory treatments for eczema: network meta-analysis. Cochrane Database Syst Rev. 2024 Aug 6;8(8): CD015064.
- Vermeulen FM, et al: The European TREatment of ATopic eczema (TREAT) Registry Taskforce survey: prescribing practices in Europe for phototherapy and systemic therapy in adult patients with moderate-to-severe atopic eczema. British Journal of Dermatology 2020; 183(Issue 6): 1073-1082.
- Musters AH, et al: Phototherapy for atopic eczema. Cochrane Database Syst Rev. 2021 Oct 28;10(10): CD013870.
- Molla A: A Comprehensive Review of Phototherapy in Atopic Dermatitis: Mechanisms, Modalities, and Clinical Efficacy. Cureus. 2024 Mar 25;16(3):e56890.
- Achten RE, et al: Ocular surface disease is common in moderate-to-severe atopic dermatitis. Clin Exp Allergy 2022; 52(6): 801-805.
- Drucker AM, et al: Systemic Immunomodulatory Treatments for Atopic Dermatitis. Living Systematic Review and Network Meta-Analysis Update. JAMA Dermatol. 2024;160(9):936-944.
- Caron AGM, et al.: MTX Consensus for AD Survey Study Group. International consensus on methotrexate dosing for patients with atopic dermatitis: An eDelphi study. J Eur Acad Dermatol Venereol. 2024 Aug 1. doi: 10.1111/jdv.20271.
- TREatment of ATopic eczema Registry Taskforce (TREAT), https://treat-registry-taskforce.org,(last accessed 27.11.2024).
- Spuls PI, et al: The International TREatment of ATopic Eczema (TREAT) Registry Taskforce: An Initiative to Harmonize Data Collection across National Atopic Eczema Photo- and Systemic Therapy Registries. J Invest Dermatol 2017; 137(9): 2014-2016.
DERMATOLOGIE PRAXIS 2024; 34(6): 34-35 (published on 13.12.24, ahead of print)