The revolution in systemic psoriasis therapy began with the introduction of anti-TNF treatment after the turn of the millennium. Since then, the chain of further new psoriasis drug launches has not broken. Experts spoke about the biologic secukinumab, future prospects for systemic psoriasis therapy and new Swiss guidelines at a symposium on the occasion of the National Psoriasis Day 2016 in Zurich.
Until now, new systemic drugs were discovered by chance because they unexpectedly proved effective against psoriasis. The anti-IL-17 antibody secukinumab is the first drug introduced in psoriasis therapy that has been specifically developed based on the understanding of psoriasis pathogenesis, reported PD Dr. Curdin Conrad, Service de dermatologie et vénérologie, CHUV, Lausanne.
After secukinumab (half-life 27 days), ixekizumab (half-life 12 days) would soon become available as another IL-17A inhibitor. The development of the IL-17RA receptor blocker brodalumab is also being intensively pursued. The different half-lives play a role in practice, because before surgery or vaccination with live vaccines, the recommended waiting time is about 5 half-lives.
Very efficient psoriasis treatment with secukinumab
In over 40% of patients, psoriasis plaques can be completely disappeared with secukinumab (PASI100 response). Previously, no such efficient psoriasis treatment had been available, the speaker said. Secukinumab was not only rapidly and strongly effective, but also maintained its efficacy for a long time (PASI100 response with secukinumab dosage of 300 mg still 43% after 3 years). The biologic is also very effective against nail infestation. The physiological function of IL-17 is that interleukin in skin and mucous membranes participates in immunological protection against extracellular pathogens (fungi, bacteria).
IL-17 is known to induce secretion of antimicrobial peptides in epithelial cells and to provide for granulocyte recruitment. Accordingly, mild to moderate Candida infections occur more frequently as side effects with anti-IL-17 therapy, which can be treated without having to discontinue the biologic. Dr. Conrad reported that repeated Candida infections are rare, usually in the same location and only in the first year. The speaker has also seen cases of Candida esophagitis at CHUV.
Future psoriasis therapies
Prof. Dr. Nikhil Yawalkar, University Department of Dermatology, Inselspital Bern, reported that the special TNF antagonist certolizumab pegol (Cimzia®, so far approved for psoriatic arthritis, rheumatoid arthritis, axial spondyloarthritis, M. Crohn) will probably soon also be approved for psoriasis therapy. It is a pegylated molecule, with the Fab portion of a recombinant humanized antibody chemically conjugated to polyethylene glycol. The half-life of the biologic, which can be injected subcutaneously every 2 or 4 weeks, is 14 days. The PASI75 response at 12 weeks was approximately 80% and the PASI90 response was nearly 50%. Certolizumab pegol does not pass the placenta and is therefore also used by rheumatologists at Inselspital in women who wish to become pregnant.
The study results on the efficacy of the IL-17A inhibitor ixekizumab, which is already approved in the EU, are very similar to those of secukinumab, the speaker said. The memorable formula 90 – 70 – 40 (for PASI75 or PASI90 or PASI100 response) applied not only to secukinumab but also to ixekizumab, he said. Over the course of 60 weeks, efficacy remains stable (PASI100 response at 60 weeks in 55% of patients) [1]. The biologic is already highly effective in the first few weeks. Patients should be informed that complete disappearance may take more time (up to 6 months) [1]. Candida infections (usually well treatable) occur in 3 to 4 cases when 100 patients are treated during one year.
The IL-17RA receptor inhibitor brodalumab (half-life 10.4 days), already approved in Japan, blocks the action of several cytokines of the IL-17 family. The formula 90 – 70 – 40 is also valid for response rates with brodalumab. The long-term results were very good (PASI100 response at 52 weeks 56%), the speaker said. Candida infections occur in 5 to 6 cases when 100 patients are treated during one year.
Swiss Guidelines for Systemic Psoriasis Therapy
Prof. Dr. Alexander Navarini, Deputy. Head of the Dermatological Polyclinic, UniversitySpital Zurich, presented the new evidence-based Swiss guidelines for the systemic treatment of psoriasis vulgaris [2]. This is an informal consensus of all psoriasis experts at major hospitals throughout Switzerland. The guidelines are based on the German S3 guidelines (updated 2011) as well as the European S3 guidelines (updated 2015) and additionally consider more recent clinical studies. Also included is useful information on specific regulatory aspects in Switzerland. The guidelines can serve as a reference for obtaining cost approvals from health insurance companies and insurers in special cases, the speaker said. Regarding the definition of moderate to severe psoriasis, the Swiss experts are of the opinion that politicians, health insurers and insurance companies should recognize a DLQI (“Dermatology Life Quality Index”) value above 10 as an official threshold, in addition to a PASI value above 10 or a BSA (“Body Surface Area”) value above 10%. The treating physician can refer to this opinion in the application for approval of the costs of a required biologic, said Prof. Navarini. In some severely affected body regions, the DLQI is the decisive criterion (e.g., scalp, genitals, palms, soles, nails, individual very persistent plaques, unquenchable pruritus with scratch damage). In the Guidelines, the Precise PASI is now considered as a more accurate method of calculating the PASI [3]. Unlike the conventional PASI, which increases in steps, the Precise PASI increases linearly. In the new score, the percentage of skin area affected is determined precisely, whereas the conventional PASI uses a gradual method (1 to 9%, 10 to 29%, etc.). With the more sensitive Precise PASI method, it is possible to reliably detect and document improvements even in deeper areas of BSA (less than 10% affected area) during the course of psoriasis treatment [3].
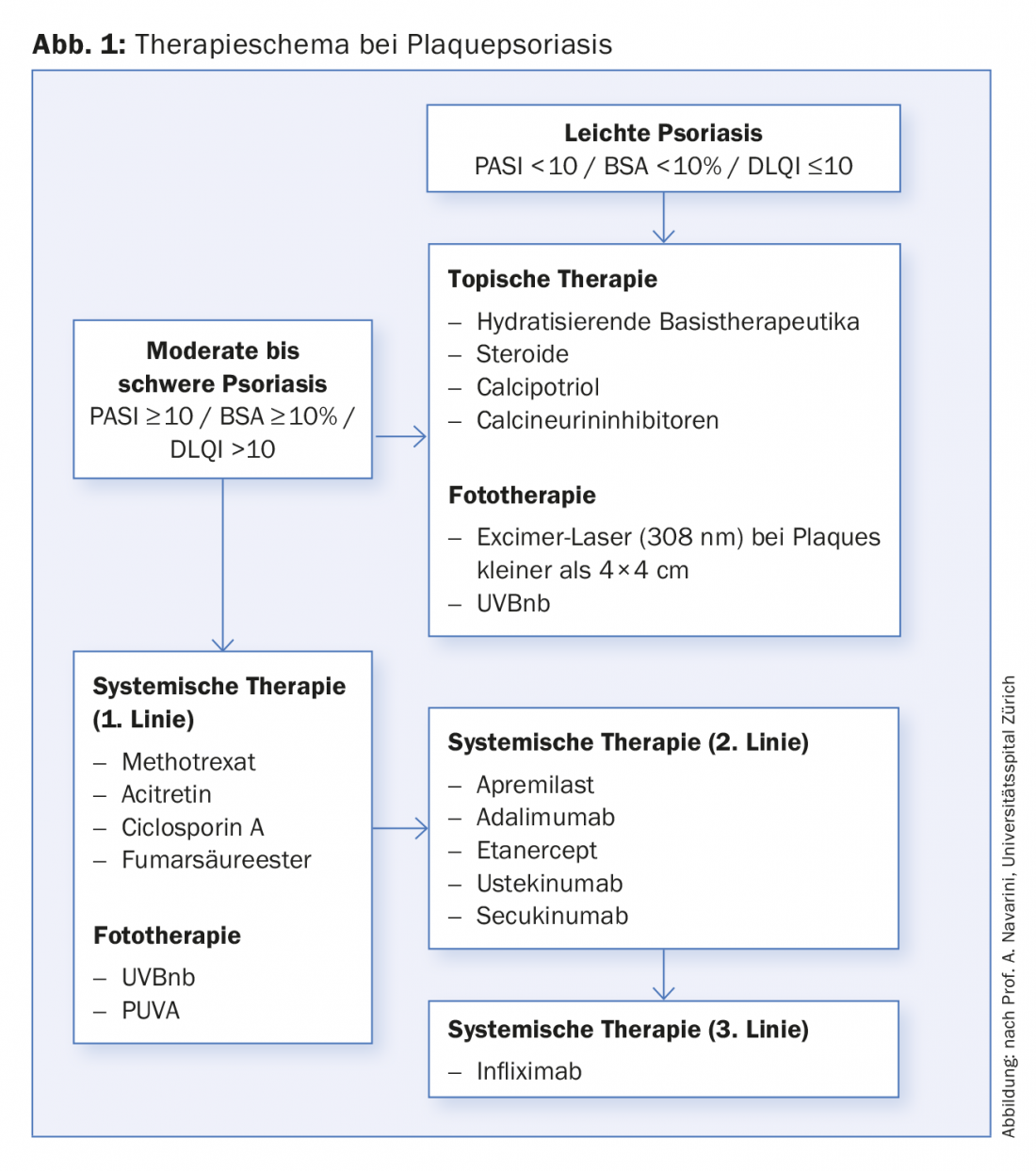
Very useful are the tables included in the guidelines on the commonly used small molecule psoriasis drugs (methotrexate, acitretin, fumaric acid ester [in der Schweiz nicht formell registriert], ciclosporin A, apremilast) and the currently available biologics (etanercept, infliximab, adalimumab, ustekinumab, secukinumab). The tables also summarize the laboratory tests recommended by the experts before starting treatment [2]. For example, prior to therapy with apremilast, the recommended screening is: HBs-Ag, HBs-Ab, HBc-Ab, HCV, HIV, creatinine, pregnancy test. Optional recommendations also include: blood count, transaminases, CRP, tuberculosis testing. The tables also show the annual costs of the drugs [2]. However, the guidelines do not contain a strict treatment algorithm. The therapeutic procedure commonly used at the University Hospital Zurich is shown in Figure 1.
Source: National Psoriasis Day, 27 October 2016, Zurich.
Literature:
- Gordon KB, et al: Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med 2016; 375: 345-356.
- Kolios AG, et al: Swiss S1 guidelines on the systemic treatment of psoriasis vulgaris. Dermatology 2016; 232: 385-406.
- Kolios AG, et al: Detection of small changes in psoriasis intensity with Precise PASI. Dermatology 2015; 230: 314-317.
DERMATOLOGIE PRAXIS 2016; 26(6): 38-40