About half of all diabetics develop chronic kidney disease. SGLT-2 inhibitors have been shown not only to favorably affect glucose metabolism, but also to protect the heart and kidneys. The nephroprotective benefit of dapagliflozin was impressively demonstrated in the DAPA-CKD study. In a recently presented secondary analysis of these trial data, the renal protective effects were found to be similar in type 2 diabetic patients with varying degrees of macroalbuminuria.
Chronic renal failure (CKD) is a severe, progressive renal impairment associated with multimorbidity and increased risk of cardiovascular events such as heart failure and premature death [1,2]. Diabetes is one of the most common causes of CKD, along with hypertension and glomerulonephritis [3]. According to international data, about 30-50% of cases of CKD are caused by diabetes [4–6]. CKD is diagnosed using the following three criteria (CGA): cause; glomerular filtration rate (GFR) G1-G5; albuminuria A1-A3. Kidney disease is considered chronic if the change in kidney structure and function persists for more than three months.
DAPA-CKD: dapagliflozin reduces progression of chronic renal failure
Dapagliflozin is the first SGLT-2 inhibitor to show meaningful benefit in chronic renal failure in both patients with and without type 2 diabetes. The corresponding empirical evidence was provided in the DAPA-CKD study. In the multicenter RCT “Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease,” more than 4000 adult patients with chronic renal failure were randomized to treatment with dapagliflozin 10 mg or placebo. The study included CKD patients with and without diabetes who had an eGFR in the range 25-75 ml/min/1.73 m2 and albuminuria. The criterion for albuminuria was a urinary albumin-to-creatinine ratio (UACR) of 200-5000 mg/g. Dapagliflozin and placebo, respectively, were administered once daily as an add-on to the standard of care. The primary composite endpoint was worsening renal function (decrease in eGFR ≥50%), onset of end-stage renal failure, and death from cardiovascular or renal causes) [7]. Secondary end points were a ≥50% reduction in eGFR, end-stage renal failure, and renal death, as well as hospitalization for heart failure (HHF), cardiovascular death, and all-cause mortality.
The main outcome of the study was that dapagliflozin reduced the risk of renal failure and hospitalization in CKD patients with and without type 2 diabetes. The topline results of the DAPA-CKD study appeared last year in the New England Journal of Medicine [8]. In the meantime, several additional analyses of the dataset were performed. The current status of the results was presented at the EASD Annual Congress 2021 by Prof. David C. Wheeler, MD, University College London (UK) [9].
| Mechanism of action SGLT-2 inhibitors: cardio- and nephroprotective effects.
In recent years, it has been repeatedly demonstrated that SGLT-2 inhibitors not only have a beneficial effect on glucose metabolism, but also have a protective effect on the heart and kidneys. The mechanism of action of SGLT-2 inhibitors is to increase urinary glucose excretion by inhibiting sodium-glucose co-transporter 2 (SGLT-2) at the kidney, which is responsible for reabsorption of glucose from the urine into the circulation. By activating the tubuloglomerular feedback mechanism in the kidney, SGLT-2 inhibitors significantly reduce albuminuria and have an organ-protective effect [11]. This results in delayed progression of kidney disease with a smaller drop in GFR over time. |
Secondary analysis shows: consistent effects across a broad spectrum of macroalbuminuria.
“We know that albuminuria is a risk marker for progression of CKD and for cardiovascular events,” said Prof. Wheeler [9]. Considering that more severe albuminuria in CKD patients is associated with increased risk of renal failure and hospitalization for heart failure, DAPA-CKD study data were categorized based on urinary albumin-to-creatinine ratio (UACL) at baseline and analyzed with respect to primary and secondary endpoints [10]. The main finding of this secondary analysis was that the efficacy and safety of dapagliflozin on renal and cardiovascular endpoints proved consistent regardless of the UACR subgroup. These results indicate that dapagliflozin is effective and safe across a broad spectrum of macroalbuminuria (Table 1).
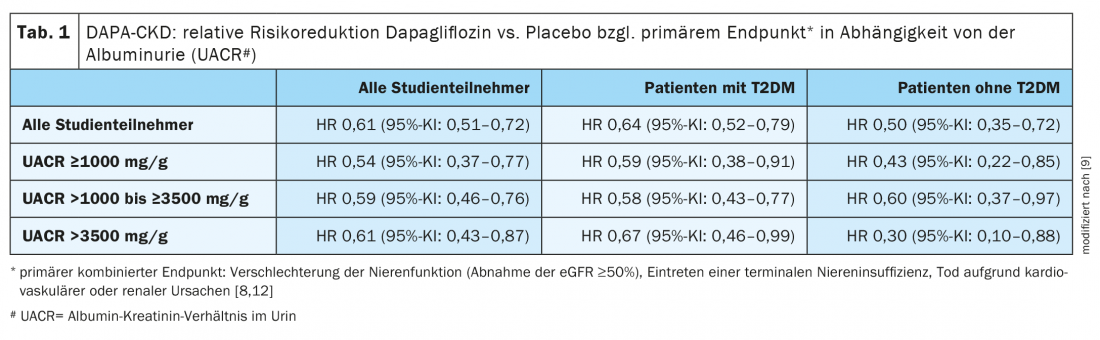
To examine the relative and absolute effects of dapagliflozin on UACR subgroups, Cox proportional hazards regression analyses were performed. At the time of randomization, 51.7%, 41.0%, and 7.3%, respectively, of the 4304 included study participants had an albumin-creatinine ratio of ≤1000, >1000 to ≤3500, and >3500 mg/g, respectively. Separate analyses of patients with and without diabetes showed that the relative risk reduction with dapagliflozin with respect to the primary endpoint was consistent across UACR subgroups (Table 1) .
The proportion of subjects with adverse events leading to discontinuation of the study intervention, including severe adverse events, proved similar across groups, regardless of UACR category.
Literature:
- Bikbov B, et al: Lancet 2020; 395(10225): 709-733.
- Segall L, et al: Biomed Res Int 2014; 2014: 937398.
- National Kidney Foundation. Kidney Disease: Causes, 2017; www.kidney.org, (last accessed Oct. 20, 2021).
- Webster AC, et al: Lancet 2017; 389: 1238-1523.
- Liyanage T, et al: Lancet 2015; 385: 1975-1982.
- IDF: Diabetes Atlas, www.diabetesatlas.org (last accessed Oct. 20, 2021).
- Heerspink HJL, et al: Nephrology Dialysis Transplantation 2020; 35 (2): 274-282.
- Heerspink HJL, et al: N Engl J Med 2020; 383: 1436-1446.
- Wheeler DC: DAPA-CKD: Updates from a Nephrology Perspective. Prof. Dr. med. David C. Wheeler, EASD Virtual Meeting 29.09.2021
- Heerspink HJL, et al: Efficacy and safety of dapagliflozin on kidney and cardiovascular outcomes by baseline albuminuria: a secondary analysis of the DAPA-CKD trial. OP 09 SGLT2 inhibitor trials, Abstract 51, EASD Annual Meeting, Diabetologia 2021; 64.
- Vallon V, Thomson SC:. Diabetologia 2017; 60: 215-225
- NCT03036150, https://clinicaltrials.gov/ct2/show/NCT03036150 (last accessed Oct. 20, 2021).
HAUSARZT PRAXIS 2022; 17(2): 36-37