Lung cancer screening carries certain risks such as unnecessary additional investigations or surgery in case of false-positive findings. Accordingly, it should be done in centers where all necessary disciplines work together. Data should be collected for quality control purposes.
Lung cancer is the most common cancer-related cause of death in much of the world. According to the WHO World Cancer Report for 2014, 13% of all cancer cases and 19.4% of cancer-related deaths are due to lung cancer [1].
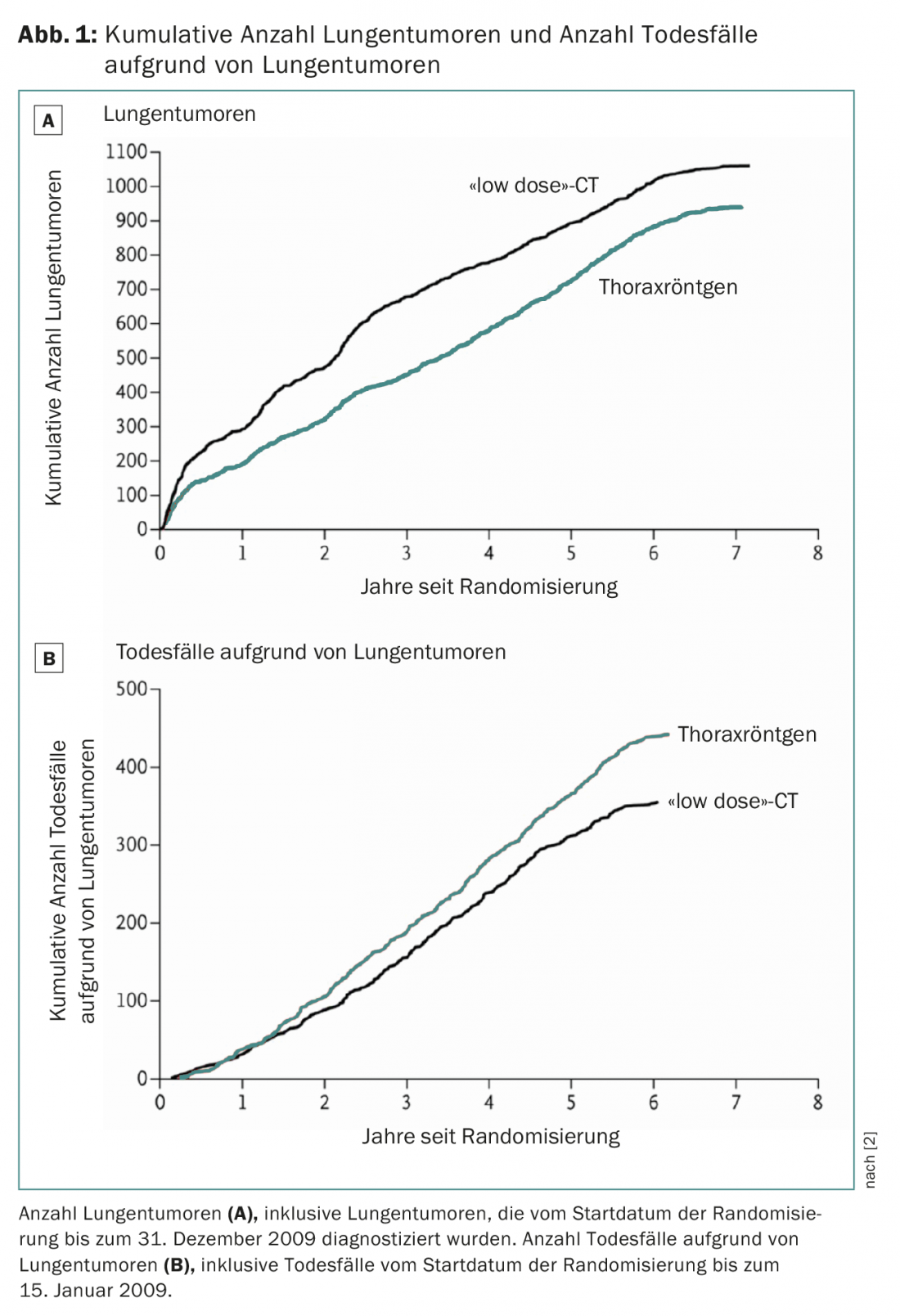
Results from the US National Lung Cancer Screening Trial (NLST) demonstrated that low-dose CT can reduce lung cancer mortality by 20% (Fig. 1) [2]. Since then, several professional societies and health care organizations have issued recommendations for CT screening (American Association for Thoracic Surgery, ATS [3]; Society of Thoracic Surgeons, STS [4]; National Comprehensive Cancer Network [5]; International Association for the Study of Lung Cancer, IASLC [6]; American Society of Clinical Oncology, ASCO [7]; and others [8–10]. In the U.S., the cost of screening is covered by insurance.
In Europe, recommendations have been developed by the ERS and ESMO, although decisions by national health organizations are pending and deferred due to the pending results of the Dutch-Belgian NELSON trial [11].
Also in Switzerland, a university group of radiologists, pulmonologists, thoracic surgeons, and epidemiologists formed in 2012 to actively address the issue of “systematic lung tumor screening in Switzerland” and in 2014 made recommendations for the introduction of lung cancer screening in a comprehensive quality-assured program in certified interdisciplinary medical centers [12]. An application for cost coverage by the mandatory health insurance was submitted in 2014 with the aim of preventing opportunistic screening and establishing systematic screening in Switzerland with clear guidelines and a central registry. This request was rejected by the FOPH due to the lack of data from the NELSON trial.
In late 2017, a group led by prominent specialists from all participating disciplines in Europe published a statement outlining the current state of lung cancer screening and the essential elements needed to develop effective European screening programs [13]. The focus of this EU Opinion is on the actual implementation of CT lung cancer screening programs in Europe by radiologists supported by epidemiologists, pulmonologists, and thoracic surgeons in the context of clinical diagnosis and treatment of lung cancer. The goal of all recommendations is to minimize the number of false-positive results, in nodes detected by screening but also in nodes not detected by screening. The need for low-dose interval CT should not be considered a false-positive test because the patient is not undergoing an invasive clinical examination and therefore the risk of physical harm is very low.
Results of European lung cancer screening studies
Several nationally funded randomized trials have already been conducted in Europe to verify the feasibility of lung cancer screening. Here, the NELSON trial, whose results are still pending, is the only randomized controlled trial in Europe that will provide data on mortality and cost-effectiveness.
Lung cancer risk prediction models
The concept of clearly defining the appropriate target population for lung cancer screening is central [14]. Selection based on age alone – as with most other cancer screening (e.g., breast and colon) – is not sufficient for lung cancer because risk factors (especially nicotine abuse) play an important role. Other risk factors to consider include respiratory disease (COPD, emphysema, bronchitis, pneumonia, and tuberculosis), tumor history, family history of lung cancer, and asbestos exposure. Several risk prediction models have been published after testing in the NLST dataset and partial validation, but to date only two patient selection models have been used in clinical trials: the modified Liverpool Lung Project (LLPv2) and Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO) model [15,16]. It appeared that NLST selection criteria could be improved if risk prediction models were implemented [17]. In addition, it showed an increase in cost-effectiveness in the high-risk groups, implying that risk prediction can also reduce the cost per life saved [18].
Harm, benefit and prerequisites
Facilities participating in screening programs need multidisciplinary teams that offer all relevant specialties (pulmonology, thoracic surgery, radiology) and where screening findings can be discussed in an interdisciplinary manner. These facilities would be required to regularly demonstrate that they meet basic standards [12,13].
The risks associated with lung cancer screening, such as overdiagnosis, surgery of benign lesions, psychological distress, and radiation damage, must be accepted before screening is implemented and can be reduced by adjusted inclusion criteria and a high level of clinical expertise. Lung cancer screening should therefore be performed only according to mandatory protocol and in screening units and centers that can ensure strict quality control and offer all necessary experts as well as a smoking cessation program. National quality assurance committees guarantee compliance with minimum technical standards and standardization of diagnostic criteria for lung lesions detected in screening (similar to the European chest examination programs). Radiation risk is likely overestimated and will decrease in the future with the introduction of the latest CT platforms with “ultra low dose” CT technology.
The number of benign resections should not exceed 10% [13]. Inclusion of board-certified thoracic surgeons in such a screening program is central to keeping the risk of unnecessary resections [4] and perioperative risk as low as possible. There are clear guidelines on this from the European Society for Thoracic Surgery (ESTS) [19], including requirements for how such a clinic should be structured and designed [20].
Algorithm for detected nodes
In the NLST study, a CT finding was considered positive if it showed a noncalcified nodule with a diameter of at least 4 mm. An alternative method is to determine the lung round heart volume [21]. The European Expert Group recommends that semi-automatically derived volume and volume doubling time should be used instead of diameter for the future management of solid foci (Fig. 2) [13].
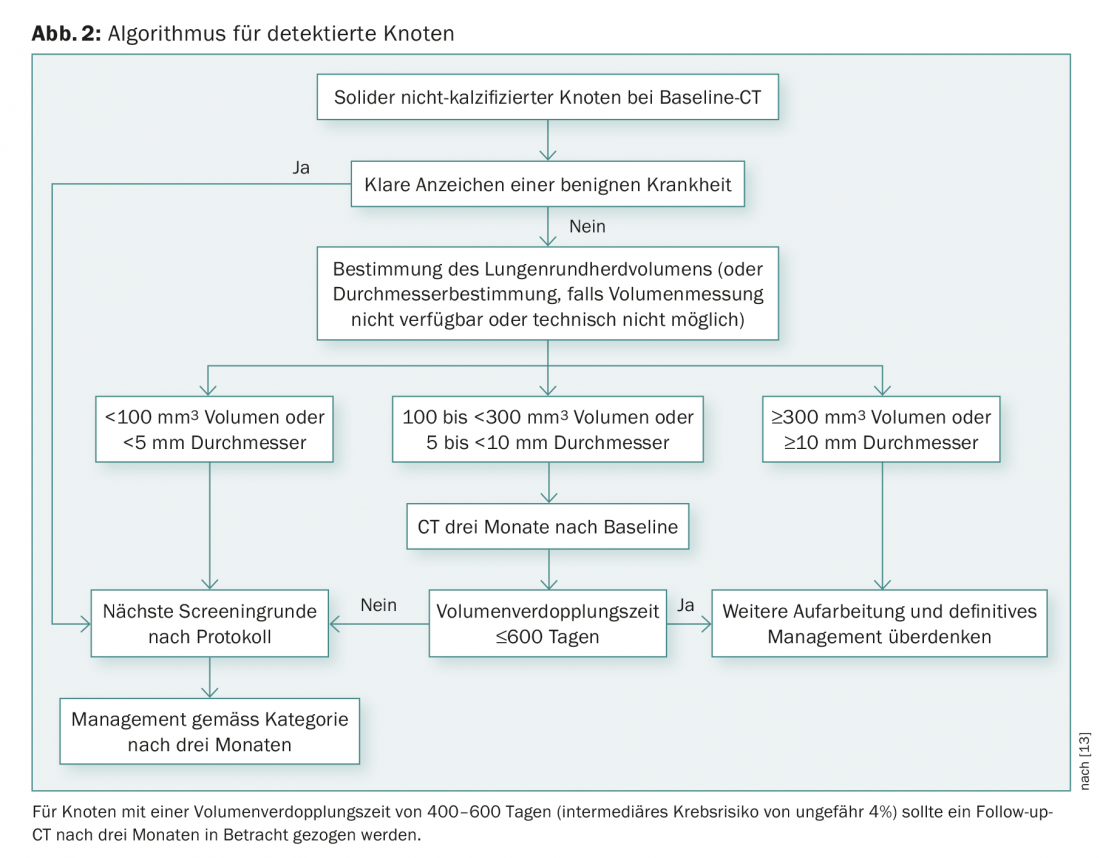
Based on current data, solid nodules with volumes less than 100 mm3 should be examined annually; nodules with volumes 100-300 mm3 should return for repeat CT after three months; volumes greater than 300 mm3 should refer the patient to a multidisciplinary team [22].
Optimal lung cancer screening intervals
The U.S. Preventive Services Task Force (USPSTF) on CT screening has recommended annual screening from age 55 years to 80 years. In a NELSON publication, a screening interval of two and a half years resulted in a significant increase in interval cancers in the fourth round of screening, providing an argument against the use of such long intervals. A detailed analysis of the cost-effectiveness of different screening scenarios showed that for almost all approaches, cost-effectiveness increased when screenings were performed annually.
Half of the participants in the NELSON study did not have a lung focus detected and had a 2-year probability of developing lung cancer of 0.4%, suggesting that a screening interval of up to 2 years could be considered for analogous risk groups in similar screening programs in a risk-stratified approach [23].
Systematic screening in Switzerland
First of all, it has to be stated again that at the present time such a screening of asymptomatic high-risk smokers is not included in the catalog of benefits of the OKP. The costs would therefore have to be borne by the patient. The situation is different when suspicious symptoms such as persistent dry cough, bloody sputum, or shortness of breath are reported and require further clarification. At the University Hospital Zurich, we offer lung cancer screenings as part of a structured algorithm for patients seeking primary or second opinion consultations in this regard. Data will be prospectively collected in a registry in the future and research projects are planned.
Based on the results of completed and ongoing lung cancer screening activities, the following requirements are necessary for the evaluation of systematic lung cancer screening within observational studies:
- Accredited medical centers with multidisciplinary expertise and access to trained specialists in (at a minimum) radiology, pulmonology, oncology, pathology, and thoracic surgery
- Integration of an established smoking cessation program
- Comprehensive screening program that covers the entire period from inclusion to follow-up to potential re-entry
- Inclusion criteria: Age between 55 and 74 years, at least 30 pack-years, and smoking cessation no more than 15 years ago if ex-smoker.
- Standardized procedure to inform and advise interested persons in a factual and independent manner whether screening with all its possible consequences should be performed (supported by individual risk assessment models)
- Standardized operating procedures for image acquisition, volumetric pulmonary round evaluation, positive screening results and their management, and monitoring of false-positive results and iatrogenic complications
- Collection and submission of lung cancer screening data to a national lung tumor screening registry.
- Regular quality check.
Take-Home Messages
- Lung cancer screening by “low dose” CT reduces lung cancer mortality by 20% in a high-risk population.
- Lung cancer screening also has risks such as unnecessary additional investigations and unnecessary surgeries in the clarification of false-positive findings.
- Accordingly, lung cancer screening should be performed in centers where all necessary disciplines (radiologists, pulmonologists, thoracic surgeons) are involved in the review of findings, and which have data collection for quality control.
- Inclusion criteria should be based on scientific data.
Literature:
- WHO: World Cancer Report. 2014.
- Aberle DR, et al: Reduced lung-cancer mortality with low-dose computed tomographic screening. The New England journal of medicine 2011; 365(5): 395-409.
- Jaklitsch MT, et al: The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg 2012; 144(1): 33-38.
- Rocco G, et al: Clinical statement on the role of the surgeon and surgical issues relating to computed tomography screening programs for lung cancer. Ann Thorac Surg 2013; 96(1): 357-360.
- Wood DE: National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin 2015; 25(2): 185-197.
- Field JK, et al: International Association for the Study of Lung Cancer Computed Tomography Screening Workshop 2011 report. J Thorac Oncol 2012; 7(1): 10-19.
- Bach PB, et al: Benefits and harms of CT screening for lung cancer: a systematic review. JAMA 2012; 307(22): 2418-2429.
- American Lung Association: Providing Guidance on Lung Cancer Screening to Patients and Physicians. www.lung.org
- Wender R, et al: American Cancer Society lung cancer screening guidelines. CA Cancer J Clin 2013; 63(2): 107-117.
- Mulshine JL, D’Amico TA: Issues with implementing a high-quality lung cancer screening program. CA Cancer J Clin 2014; 64(5): 352-363.
- van Klaveren RJ, et al: Management of lung nodules detected by volume CT scanning. The New England journal of medicine 2009; 361(23): 2221-2229.
- Frauenfelder T, et al: Early detection of lung cancer: a statement from an expert panel of the Swiss university hospitals on lung cancer screening. Respiration; international review of thoracic diseases 2014; 87(3): 254-264.
- Oudkerk M, et al: European position statement on lung cancer screening. The Lancet Oncology 2017; 18(12): e754-e766.
- Tammemagi MC, et al: Participant selection for lung cancer screening by risk modeling (the Pan-Canadian Early Detection of Lung Cancer [PanCan] study): a single-arm, prospective study. The Lancet Oncology 2017; 18(11): 1523-1531.
- Cassidy A, et al: The LLP risk model: an individual risk prediction model for lung cancer. British journal of cancer 2008; 98(2): 270-276.
- Tammemagi MC, Mayo JR, Lam S: Cancer in pulmonary nodules detected on first screening CT. The New England journal of medicine 2013; 369(21): 2060-2061.
- Katki HA, et al: Development and Validation of Risk Models to Select Ever-Smokers for CT Lung Cancer Screening. Jama 2016; 315(21): 2300-2311.
- Tammemagi MC, et al: Selection criteria for lung-cancer screening. The New England journal of medicine 2013; 368(8): 728-736.
- Pedersen JH, et al: Recommendations from the European Society of Thoracic Surgeons (ESTS) regarding computed tomography screening for lung cancer in Europe. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery 2017; 51(3): 411-420.
- Brunelli A, et al: European guidelines on structure and qualification of general thoracic surgery. European journal of cardio-thoracic surgery: official journal of the European Association for Cardio-thoracic Surgery 2014; 45(5): 779-786.
- Henschke CI, et al: Early Lung Cancer Action Project: overall design and findings from baseline screening. Lancet (London, England) 1999; 354(9173): 99-105.
- Horeweg N, et al: Lung cancer probability in patients with CT-detected pulmonary nodules: a prespecified analysis of data from the NELSON trial of low-dose CT screening. The Lancet Oncology 2014; 15(12): 1332-1341.
- Pastorino U, et al: Annual or biennial CT screening versus observation in heavy smokers: 5-year results of the MILD trial. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation 2012; 21(3): 308-315.
InFo ONCOLOGY & HEMATOLOGY 2018; 6(2): 14-17.