Around 200,000 patients in Switzerland live with a diagnosis of heart failure. Up to 10% of them develop progressive symptoms. At what point is severe heart failure diagnosed and what can be done?
In 2018, the Heart Failure Association of the European Society of Cardiology published a position paper addressing severe heart failure [1]. This text is mostly a summary of the recommendations published there.
In Switzerland, 150,000-200,000 patients are living with a diagnosis of heart failure [2]. Despite advances in heart failure therapy in recent years and new drugs, such as sacubitril/valsartan, approximately 5-10% of patients with heart failure will develop progressive symptoms and suffer from progressive, severe heart failure [3–5]. The number of patients with severe heart failure will continue to increase with improved survival and rising incidence of heart failure. It is critical that primary care providers and office-based cardiologists recognize and diagnose severe heart failure and continue to refer patients to a tertiary heart failure center at the appropriate time. Only in this way can further therapeutic options, including listing for heart transplantation or the use of mechanical circulatory support systems (MCS), be planned early and successfully implemented.
Definition of severe heart failure and prognosis determination.
There are several definitions of severe heart failure in the literature [5–8]. Because of its completeness and clinical applicability, we consider the recently published Heart Failure Associaton (HFA)-ESC definition to be very useful for clinical practice. All of the following criteria must be met despite optimal heart failure therapy
- Severe persistent symptoms of heart failure (NYHA class III or IV).
- Severe cardiac dysfunction defined by a reduction in LVEF <30%, isolated right ventricular failure, or inoperable severe valve abnormalities or congenital abnormalities or persistently high (alternatively increasing) BNP/NT-proBNP values and severe diastolic dysfunction or structural LV abnormalities according to the ESC definition of HFpEF and HFmrEF.
- Episodes of pulmonary congestion or systemic congestion requiring high-dose intravenous therapy with diuretics (or diuretic combinations) or episodes of low-output requiring inotropics or vasoactive medications or malignant arrhythmias resulting in at least one unplanned presentation or hospitalization in the past 12 months
- Severe limitation of physical performance, optimally objectified by a 6-minute walk test below 300 meters or spiroergometry with maximal O2 uptake of (pVO2 <12-14 mL/kg/min) with suspected cardiac etiology
In addition, advanced heart failure is characterized by systemic organ dysfunction (renal failure, cardiac cachexia, hepatic failure) and/or pulmonary hypertension. In this sense, a cardio-renal syndrome or a weight loss of 6% of body weight within 6 months (definition of cardiac cachexia) are indicators of severe heart failure. Cardiac-related pulmonary hypertension should be documented early and interpreted as evidence of advanced heart failure. If this is detected too late, there is a risk that its extent may be a contraindication to heart transplantation. Also, the associated deterioration of right heart function may preclude further therapy with a left ventricular assist device.
Concomitant diseases are very often associated with heart failure. In defining severe heart failure, criteria 1 and 4 should be considered met even if there is cardiac dysfunction according to criterion 2 but much of the limitation is explained by comorbidity (eg, pulmonary disease). Comorbidities such as diabetes, pulmonary diseases (COPD, sleep apnea), renal insufficiency, anemia, iron deficiency, or chronic rheumatic diseases significantly influence the prognosis of heart failure and should be treated as best as possible [9]. Therefore, comorbidities should definitely be included in the overall assessment and prognosis, as they may reflect the severity of heart failure on the one hand and may also be possible contraindications for heart replacement therapies (heart transplantation, cardiac assist devices) on the other hand. End-organ dysfunction in particular has a negative impact on the overall prognosis. Further investigations to predict possible reversibility of end-organ dysfunction after transplantation or mechanical cardiac support (eg, in renal failure) are useful but often remain inconclusive.
Regular risk stratification is essential in severe heart failure in order not to miss the optimal time for referral to the heart failure center and to initiate further clarification, treatment and follow-up. Prediction of prognosis and the associated risk stratification cannot be performed on the basis of a single parameter, but requires the inclusion of different prognosis-sensitive variables. Several such multivariable scores have been clinically validated and are widely used. The Heart Failure Survival Score (HFSS) and the Seattle Heart Failure Model (SHFM) are among the most commonly used scores in clinical practice [10,11]. Other scores include the (MECKI) score (Metabolic Exercise test data combined with Cardiac and Kidney indexes score) and the MAGGIC score Meta-Analysis Global Group in Chronic Herat Failure [12–15].
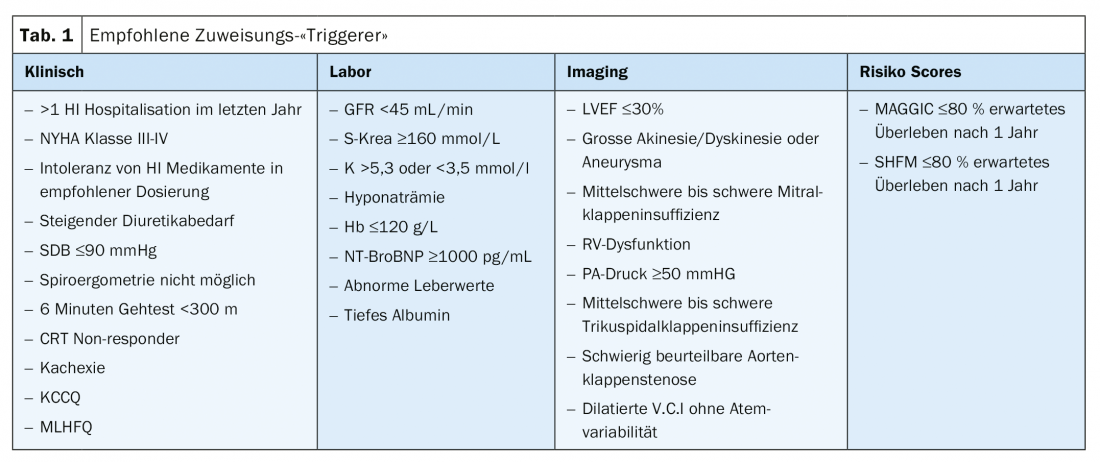
Although the achievement of a certain score (e.g., HFSS) is associated with a recommendation for evaluation of cardiac replacement therapy, there is currently no threshold at which referral to the heart failure center should occur. Table 1 summarizes the clinical, laboratory chemistry, imaging, and risk score triggers listed by the HFA-ESC that should lead to referral to a heart failure center. All too often, unfortunately, patients are referred too late. In general, if the definition of severe heart failure is met, a center should be contacted.
Spiroergometry and 6-minute walk test
Spiroergometry is a key investigation for risk stratification of outpatients with heart failure. In addition to prognostic information, objective data on global performance, cardio-pulmonary limitation, and cardiovascular reserve are generated.
A maximal oxygen uptake (pVO2) ≤12 ml/kg/min (≤14 ml/kg/min without beta-blocker therapy) is considered an indication for listing for cardiac transplantation or MCS according to the guidelines [16]. Women who achieve ≤50% of maximal oxygen uptake if younger than 50 years could also be evaluated for heart transplantation [16]. If the respiratory equivalent for carbon dioxide (V E/VCO2) is greater than 35, this is an indication of a poor prognosis.
The 6-minute walk test is a submaximal exercise test, unlike spiroergometry, which is a maximal exercise test. The results of the studies regarding correlation with survival are not consistent [17–20]. If spiroergometry is not possible, the 6-minute walk test is a valid alternative. A walking distance of <300 meters thereby identifies patients with severe power intolerance.
Treatment strategies for patients with severe heart failure
There are only two long-term treatment strategies for severe heart failure unless a palliative approach is chosen. These are heart transplantation or a mechanical circulatory support system (MCS). Mechanical short-term circulatory support systems and intravenous vasoactive medications are available as bridging solutions. The basic therapy for overhydration is diuretics.
Treatment of overwatering
Loop diuretics are the basic therapy for hypervolemia. In severe heart failure, there is often diuretic resistance and increasing renal insufficiency. Long-term use of diuretics may result in various renal adaptive mechanisms, such as hypertrophy and hyperfunction in the distal nephron, and increased renin secretion. In addition, an increase in uremic anions and proteinuria may impair the efficacy of diuretics [21]. In clinical practice, sequential nephron blockade, a combination of a loop diuretic and a thiazide diuretic (e.g., Metolazone), is often used to break diuretic resistance. However, there is little evidence to support this approach.
With so-called ultrafiltration, fluid can be removed from the blood through a semi-permeable membrane via a dialysis machine. In the absence of response to peroral diuretics, conversion to intravenous administration is primarily recommended. This should be started with a higher dosage and successively increased until sufficient diuresis is achieved. If this is not achieved, a diuretic combination with sequential nephron blockade is recommended as the next step, and only if these measures also fail should ultrafiltration be considered in selected cases [7,9].
Intravenous vasoactive drugs
These play a role especially in the acute situation in patients with evidence of low output syndrome and hypoperfusion. In addition, there is an indication in selected patients as a bridge until implantation of an MCS or performance of a heart transplant. Although inotropics may improve hemodynamic parameters, studies overwhelmingly show no improvement in outcome. Some studies even suggest a worsening of prognosis [22–24]. Therefore, long-term use of inotropics should be avoided. Only if no other therapeutic options are considered, sequential therapy with inotropics can be used as a palliative measure in selected cases [25,26].
Mechanical cycle support systems: short-time systems
Short-term mechanical circulatory support systems are used in the acute phase of cardiogenic shock. They allow a window of time during which cardiac function can recover through maximum unloading. In addition, one can also wait to see the course of recovery of other organ systems, such as neurological function after cardiovascular arrest. However, if there is no improvement in cardiac function, short-term systems may provide “a bridge” to implantation of a long-term ventricular assist device (VAD) or heart transplantation, should that option be chosen. There are various mechanical circuit support systems which can be applied for a limited time. The intra-aortic balloon pump (IABP) is implanted percutaneously via catheter. A balloon is implanted in the descending aorta and inflated each time during diastole. This increases diastolic pressure in the aortic root, resulting in improved coronary perfusion. Deflation of the balloon leads to a reduction in afterload and thus decreases oxygen consumption. Currently, IABP is used mainly for cardiogenic shock in ischemic heart disease by some centers, although the evidence for improvement in mortality has not been shown [1,27].
An Impella is an axial intravascular pump that can also be implanted via catheter. It can transport up to 5 liters of blood per minute from the left ventricle to the ascending aorta, thus relieving the left ventricle. Hemodynamics are improved and filling pressure is lowered; at the same time, coronary perfusion pressure is increased.
Although no clear data regarding mortality improvement have been collected to date, a small registry study showed that using a standardized protocol with early hemodynamic support using Impella CP in cardiogenic shock may be associated with improved outcome and lower mortality [28].
In extracorporeal membrane oxygenation (ECMO), blood is oxygenated outside the body through a membrane in a special heart-lung machine. In addition to complete respiratory support, the ECMO device includes an axial pump so that flows up to 6 L/min can be achieved. Peripheral veno-arterial ECMO can be implanted by an interventional cardiologist using the Seldinger technique and can maintain circulation in a failing heart and support oxygenation.
The hemodynamic effects of ECMO are not physiologic. First, the preload of the heart is reduced by draining the blood from the venous side. On the other hand, ejection of oxygenated blood with a flow of 4-6 L/min into the aorta leads to an increase in left ventricular afterload, which may result in an increase in end-diastolic left ventricular volume and filling pressure, depending on cardiac dysfunction. To prevent pulmonary edema in this situation, an Impella, for example, can also be implanted to relieve the left ventricle [29]. Similar to Impella, ECMO can be used as a “bridge to transplantation” in end-stage severe heart failure or as a “bridge to decision” in cardiogenic shock.
Long-term management of severe heart failure
When heart failure symptoms can no longer be controlled or end-organ dysfunction is imminent, advanced heart failure therapies are indicated. One prerequisite, of course, is that drug and device therapy has been optimized and exhausted according to the guidelines. In addition, patients who have an indication for revascularization should be revascularized and patients with valvular cardiopathy should receive valve replacement if indicated.
Heart transplant
Patients with severe, refractory heart failure without a treatable cause are potentially candidates for heart transplantation if conventional treatment alternatives have been exhausted. The risk assessed in the risk stratification should result in a mortality of at least >20% for the following 12 months [30]. In addition, it should be ensured that heart transplantation significantly prolongs the patient’s survival and substantially improves the quality of life. Candidates for heart transplantation should be motivated, emotionally stable, and demonstrate high compliance and adherence to therapy. An evaluation of possible comorbidities is an important part of the preliminary investigations to estimate the outcome of transplantation [16,31]. Contraindications to heart transplantation are listed in Table 2.
Pretransplant evaluation includes a complete disease history, physical status, spiroergometry, left-to-right cardiac catheterization, evaluation of peripheral arterial disease, assessment of frailty, and nutrition status. Furthermore, organ functions (kidney, liver, lung) must be assessed and screening for tumor disease and active infections is performed. Prognostic scores should be calculated and further investigations should be performed depending on the presence of co-morbidities [16]. In addition, a complete psychosocial assessment is performed [32].

The first heart transplant was performed in 1967 [33]. In Switzerland, 50 heart transplants were performed in 2018 [35]. The median survival is 12.5 years [34]. The most common causes of long-term mortality are graft failure, infection, and multiorgan failure [34]. The risk of lethal acute graft failure is greatest within the first 30 days after transplantation. Infectious complications with fatal outcomes are most common within the first 12 months due to high doses of immunosuppressants, including steroids. The risk of a relevant cellular rejection reaction decreases significantly after two years. In the long-term, mortality in the setting of tumor disease, renal failure, and transplant vasculopathy becomes more important [34].
Mechanical long-term support
A mechanical ventricular assist device (VAD) is used to support the left ventricle (LVAD), the right ventricle (RVAD), or both ventricles (BiVAD). Studies show improved survival and quality of life in patients with severe, refractory heart failure [9]. A VAD can be used as a “bridge to transplantation” while waiting for a transplant. If a patient is not a transplant candidate, for example because of age, a VAD can be used as a destination therapy. Relevant increased pulmonary vascular resistance or severe renal insufficiency are contraindications for heart transplantation but not for VAD implantation. Both pulmonary vascular resistance and severe renal insufficiency may improve with VAD therapy [36,37]. In these cases, VAD therapy is used as a “bridge to transplant candidacy.” The same applies to potentially curatively treated tumor diseases, where tumor freedom of at least 5 years should be shown before possible heart transplantation. In rare cases (eg, fulminant myocarditis), VAD therapy can be used as a “bridge to recovery.”
In the current absence of an adequate solution for long-term right ventricular or biventricular cardiac support therapy, severe right ventricular failure remains a contraindication for LVAD implantation [38].
Palliative therapy
Of all patients with severe heart failure, few are eligible for heart transplantation or VAD therapy. Once all treatment strategies have been exhausted, the goals of treatment in end-stage severe heart failure are changed from prolonging life to controlling symptoms and optimizing quality of life [39]. Conventional, purely internal medicine-cardiology therapy is often insufficient to relieve the patient’s suffering in this situation, and multidisciplinary treatment with the involvement of palliative care physicians is recommended. The PAL-HF study showed that an interdisciplinary palliative approach resulted in improvements in quality of life and anxiety and depression symptoms compared with standard therapy [40]. Access to palliative treatment concepts should therefore be low-threshold for all patients with severe heart failure. It is also recommended that a detailed living will be drawn up at an early stage. If not done, this should be done at the latest before intensive medical therapies. The patient’s individual wishes regarding life-prolonging measures, including the activity status of an implanted defibrillator, should be regularly discussed and adapted to the expected course of the disease and documented accordingly [41]. If possible, the decision of when to discontinue advanced heart failure therapies (ICD, VAD therapy, immunosuppression) should be left to the patient. If he or she is unable to make this decision, the decision should be made by family members or caregivers or by an ethical committee of the hospital.
In summary, the top priority is recognition of severe heart failure and early referral to a tertiary heart failure center. Adequate risk stratification including established risk scores, spiroergometry, and right heart catheterization should be performed at regular intervals by the heart failure center. The treatment options for severe heart failure have improved significantly, particularly due to significant technological advances in long-term ventricular assist devices (VADs). Today, cardiac support systems can also be offered to older patients with good therapeutic success. Heart transplantation remains the gold standard therapy. However, this remains a rarity due to the general shortage of organs. Linkage to a palliative care team should occur early and can significantly improve the quality of life for affected patients and their families.
Take-Home Messages
- Detect severe heart failure
- Early referral to a tertiary heart failure center to initiate further evaluation, treatment, and follow up
- Early connection to a palliative care team
Literature:
- Crespo-Leiro MG, et al. “Advanced heart failure: a position statement of the Heart Failure Association of the European Society of Cardiology, Eur J Heart Fail, 20(11): 1505-1535, Nov. 2018, doi: 10.1002/ejhf.1236.
- Swiss Heart Foundation: “The Challenge of Heart Failure” [Online]. Available: www.swissheart.ch/de/forschung/medizinische-fortschritte/herzinsuffizienz.html
- Xanthakis V, et al: Prevalence, Neurohormonal Correlates, and Prognosis of Heart Failure Stages in the Community, JACC Heart Fail, 4(10): 808-815, Jun. 2016, doi: 10.1016/j.cardfail.2016.03.003.
- Bjork JB, Alton KK, Georgiopoulou VV, et al: Defining Advanced Heart Failure: A Systematic Review of Criteria Used in Clinical Trials, J Card Fail, 22(7): 569-577, Jul. 2016, doi: 10.1016/j.cardfail.2016.03.003.
- Fang JC, et al: Advanced (stage D) heart failure: a statement from the Heart Failure Society of America Guidelines Committee, J Card Fail 21(6): 519-534, Jun. 2015, doi: 10.1016/j.cardfail.2015.04.013.
- Metra M, et al: Advanced chronic heart failure: A position statement from the Study Group on Advanced Heart Failure of the Heart Failure Association of the European Society of Cardiology, Eur J Heart Fail 9(6-7): 684-694, 2007, doi: 10.1016/j.ejheart.2007.04.003.
- Yancy CW, et al: 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines, Circulation 128(16), 1810-1852, Oct. 2013, doi: 10.1161/CIR.0b013e31829e8807.
- Hunt SA, et al: 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation, J Am Coll Cardiol 53(15): e1-e90, Apr. 2009, doi: 10.1016/j.jacc.2008.11.013.
- Ponikowski P, et al: 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution, Eur J Heart Fail 18(8): 891-975, Aug. 2016, doi: 10.1002/ejhf.592.
- Aaronson KD, Schwartz JS, Chen TM, et al: Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation, Circulation, 95(12): 2660-2667, Jun. 1997, doi: 10.1161/01.cir.95.12.2660.
- Levy WC, et al: The Seattle Heart Failure Model: prediction of survival in heart failure, Circulation 113(11): 1424-1433, Mar. 2006, doi: 10.1161/CIRCULATIONAHA.105.584102.
- Agostoni P, et al: Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: a multiparametric approach to heart failure prognosis, Int J Cardiol 167(6): 2710-2718, Sep 2013, doi: 10.1016/j.ijcard.2012.06.113.
- Agostoni P, et al: Multiparametric prognostic scores in chronic heart failure with reduced ejection fraction: a long-term comparison, Eur. J Heart Fail 20(4): 700-710, Apr. 2018, doi: 10.1002/ejhf.989.
- Corra U, et al: The metabolic exercise test data combined with Cardiac And Kidney Indexes (MECKI) score and prognosis in heart failure. A validation study, Int J Cardiol 203: 1067-1072, Jan. 2016, doi: 10.1016/j.ijcard.2015.11.075.
- Pocock SJ, et al: Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies, Eur Heart J 34(19): 1404-1413, May 2013, doi: 10.1093/eurheartj/ehs337.
- Mehra MR, et al: The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update, J. Heart Lung Transplant 35(1): 1-23, Jan. 2016, doi: 10.1016/j.healun.2015.10.023.
- Alahdab MT, Mansour IN, Napan S, Stamos TD: Six minute walk test predicts long-term all-cause mortality and heart failure rehospitalization in African-American patients hospitalized with acute decompensated heart failure, J Card Fail 15(2): 130-135, Mar. 2009, doi: 10.1016/j.cardfail.2008.10.006.
- Guazzi M, Dickstein K, Vicenzi M, Arena R: Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: a comparative analysis on clinical and prognostic insights, Circ Heart Fail 2(6): 549-555, Nov. 2009, doi: 10.1161/CIRCHEARTFAILURE.109.881326.
- Hulsmann M, et al: Prediction of outcome by neurohumoral activation, the six-minute walk test and the Minnesota Living with Heart Failure Questionnaire in an outpatient cohort with congestive heart failure, Eur Heart J 23(11): 886-891, Jun. 2002, doi: 10.1053/euhj.2001.3115.
- Wolsk E, et al: Resting and exercise haemodynamics in relation to six-minute walk test in patients with heart failure and preserved ejection fraction, Eur J Heart Fail 20(4): 715-722, Apr. 2018, doi: 10.1002/ejhf.976.
- Costanzo MR, et al: Extracorporeal ultrafiltration for fluid overload in heart failure: current status and prospects for further research, J Am Coll Cardiol 69(19): 2428-2445, May 2017, doi: 10.1016/j.jacc.2017.03.528.
- Cuffe MS, et al: Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial, JAMA 287(12): 1541-1547, Mar. 2002, doi: 10.1001/jama.287.12.1541.
- O’Connor CM, et al: Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (FIRST), Am Heart J 138(1) Pt 1: 78-86, Jul. 1999, doi: 10.1016/s0002-8703(99)70250-4.
- Packer M, et al: Effect of levosimendan on the short-term clinical course of patients with acutely decompensated heart failure, JACC Heart Fail 1(2): 103-111, Apr. 2013, doi: 10.1016/j.jchf.2012.12.004.
- Comín-Colet J, et al: Efficacy and safety of intermittent intravenous outpatient administration of levosimendan in patients with advanced heart failure: the LION-HEART multicentre randomised trial, Eur J Heart Fail 20(7): 1128-1136, Jul. 2018, doi: 10.1002/ejhf.1145.
- Altenberger J, et al: Efficacy and safety of the pulsed infusions of levosimendan in outpatients with advanced heart failure (LevoRep) study: a multicentre randomized trial, Eur J Heart Fail 16(8): 898-906, Aug. 2014, doi: 10.1002/ejhf.118.
- Unverzagt S, et al: Intra-aortic balloon pump counterpulsation (IABP) for myocardial infarction complicated by cardiogenic shock, Cochrane database Syst Rev 3: CD007398-CD007398, Mar. 2015, doi: 10.1002/14651858.CD007398.pub3.
- Costanzo MR, et al: Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure, J Am Coll Cardiol 49(6): 675-683, Feb. 2007, doi: 10.1016/j.jacc.2006.07.073.
- Pappalardo F, et al: Concomitant implantation of Impella® on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock, Eur J Heart Fail 19(3): 404-412, Mar. 2017, doi: 10.1002/ejhf.668.
- Ammirati E, et al: Current indications for heart transplantation and left ventricular assist device: a practical point of view, Eur J Intern Med 25(5): 422-429, Jun. 2014, doi: 10.1016/j.ejim.2014.02.006.
- Lund LH, et al: The Registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Heart Transplantation Report-2016; Focus Theme: Primary Diagnostic Indications for Transplant, J Heart Lung Transplant 35(10): 1158-1169, Oct. 2016, doi: 10.1016/j.healun.2016.08.017.
- Lund LH, et al: The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report-2014; focus theme: retransplantation, J Heart Lung Transplant 33(10): 996-1008, Oct. 2014, doi: 10.1016/j.healun.2014.08.003.
- Barnard CN: The operation. A human cardiac transplant: an interim report of a successful operation performed at Groote Schuur Hospital, Cape Town, S Afr Med J 41(48): 1271-1274, Dec. 1967.
- Chambers DC, et al: The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; Focus theme: donor and recipient size match, J Heart Lung Transplant 38(10): 1042-1055, Oct. 2019, doi: 10.1016/j.healun.2019.08.001.
- Federal Office of Public Health, FOPH: Figures on organ donation and transplantation in Switzerland. [Online]. Available: www.bag.admin.ch/bag/de/home/zahlen-und-statistiken/zahlen-fakten-zu-transplantationsmedizin/zahlen-fakten-zur-spende-und-transplantation-von-organen.html#-1057919152.
- Hasin T, et al: Changes in renal function after implantation of continuous-flow left ventricular assist devices, J Am Coll Cardiol 59(1): 26-36, Jan. 2012, doi: 10.1016/j.jacc.2011.09.038.
- Mikus E, et al: Reversibility of fixed pulmonary hypertension in left ventricular assist device recipients, Eur J Cardiothorac Surg 40(4): 971-977, Oct. 2011, doi: 10.1016/j.ejcts.2011.01.019.
- Harjola VP, et al: Contemporary management of acute right ventricular failure: a statement from the Heart Failure Association and the Working Group on Pulmonary Circulation and Right Ventricular Function of the European Society of Cardiology, Eur J Heart Fail 18(3): 226-241, Mar. 2016, doi: 10.1002/ejhf.478.
- Whellan DJ, et al: End-of-life care in patients with heart failure, J Card Fail 20(2): 121-134, Feb. 2014, doi: 10.1016/j.cardfail.2013.12.003.
- Rogers JG, et al: Palliative Care in Heart Failure: The PAL-HF Randomized, Controlled Clinical Trial, J Am Coll Cardiol, vol. 70, no. 3, pp. 331-341, Jul. 2017, doi: 10.1016/j.jacc.2017.05.030.
- Bayoumi E, Sheikh F, Groninger H: Palliative care in cardiac transplantation: an evolving model, Heart Fail Rev 22(5): 605-610, Sep. 2017, doi: 10.1007/s10741-017-9613-8.
CARDIOVASC 2020; 19(1): 6-11