Diroximel fumarate/DRF is a novel oral fumarate for patients with relapsing-remitting multiple sclerosis (RRMS) that was granted health insurance approval in Switzerland on November 1, 2021, and has been available since then [3,4]. In a direct comparison with dimethyl fumarate/DMF, DRF was shown to have significantly improved tolerability.
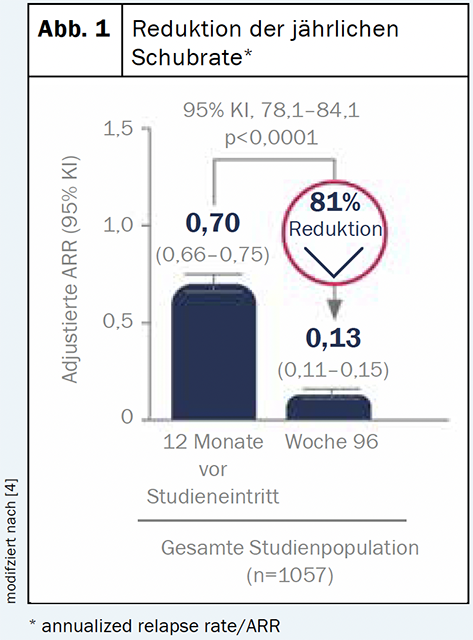
Diroximelfumarate (Vumerity™) is a substance that is converted in the body to the active metabolite monomethyl fumarate. This corresponds to the same active metabolite as in dimethyl fumarate (Tecfidera®) [4]. Therefore, the benefit and safety profiles of the two compounds are expected to be similar [4]. An interim analysis of the ongoing phase III EVOLVEMS-1 trial showed a consistent benefit-risk profile to previous findings: After two years, clinical and radiographic measures were significantly reduced from baseline [2] (Fig. 1). The low rate (<1%) of gastroenterology-related treatment discontinuations also suggests that DRF is a better tolerated option from a gastroenterology perspective [5].
Improved gastrointestinal tolerability profile
This was confirmed in the EVOLVE-MS-2 study in a direct comparison [1,2]. The objective of the double-blind phase III study was to evaluate the gastrointestinal tolerability of DRF and DMF over 5 weeks in patients with relapsing-remitting multiple sclerosis. For this, 462 mg DRF or 240 mg DMF – the two bioequivalent doses – were administered twice daily to patients with relapsing-remitting multiple sclerosis. The primary endpoint was the number of days with an IGISIS (Individual Gastrointestinal Symptom and Impact Scale) intensity score ≥2. Other endpoints included severity of gastrointestinal symptoms as measured by IGISIS/GGISIS (Global Gastrointestinal Symptom and Impact Scale) and safety/tolerability assessment. It was shown that there was a statistically significant reduction (46%) in the number of days with serious gastrointestinal events in patients treated with DRF compared with DMF. Lower rates of gastrointestinal adverse events (including diarrhea, nausea, vomiting, and abdominal pain) were observed (34.8% vs. 49.0%). This also resulted in fewer patients discontinuing treatment due to adverse events (1.6% vs. 5.6%) and gastrointestinal side effects (0.8% vs. 4.8%). The different chemical structure of DRF is thought to cause less irritation in the GI tract than DMF due to lower production of methanol (a GI-irritating procomponent) and lower reactivity with presystemic off-target proteins or receptors [1,6].
Flush diseases in focus
A particular focus on the area of flushing disorders was provided by a post-hoc analysis [7]. To present the frequency, severity, and duration of flush and flush-related adverse events under DRF compared with DMF over 5 weeks. The mechanism of DMF-induced flush is not fully understood, but it may be mediated, at least in part, by prostaglandin D2 (PGD2). During the phase III EVOLVE-MS-2 study, the incidence of flushing was lower with DRF than with DMF (46% vs. 55%). All flushes were mild or moderate in DRF, and severe flushes occurred in 5 patients (2%) in DMF . In the study investigated, under DRF (Vumerity
TM
), no patient discontinued treatment due to flushing. In the ongoing EVOLVEMS-1 trial, the rate of dropout due to flushing was <1%. This is in contrast to the 4% of DMF-treated patients who reported flushing as a treatment discontinuation in the DEFINE/CONFIRM studies [8–10].
Literature:
- Naismith RT, et al: Diroximel Fumarate Demonstrates an Improved Gastrointestinal Tolerability Profile Compared with Dimethyl Fumarate in Patients with Relapsing-Remitting Multiple Sclerosis: Results from the Randomized, Double-Blind, Phase III EVOLVEMS-2 Study. CNS Drugs 2020; 34: 185-196.
- Wundes A, et al: Improved gastrointestinal profile with diroximel fumarate is associated with a positive impact on quality of life compared with dimethyl fumarate: results from the randomized, double-blind, phase III EVOLVE-MS-2 study. Ther Adv Neurol Disord 2021; 14: 1-14.
- Piehl F, et al: Current and emerging disease modulatory therapies and treatment targets for multiple sclerosis, J Intern Med 2021; 289(6): 771 – 791.
- Technical Information Vumerity™, as of August 2021. www.swissmedicinfo.ch
- Wray S, et al: Diroximel Fumarate in Patients With Relapsing-Remitting Multiple Sclerosis: Interim Safety and Efficacy Results From the Phase 3 EVOLVE-MS-1 Study. 37th Congress of the European Committee for Treatment & Research in Multiple Sclerosis | October 13-15, 2021. Poster: P739.
- Palte MJ, et al. Improving the Gastrointestinal Tolerability of Fumaric Acid Esters: Early Findings on Gastrointestinal Events with Diroximel Fumarate in Patients with Relapsing-Remitting Multiple Sclerosis from the Phase 3, Open-Label EVOLVE-MS-1 Study. Erw Ther 2019; 36(11): 3154-3165.
- Singer BA, et al. Flushing and Flushing-Related Adverse Events With Diroximel Fumarate in Patients With Relapsing-Remitting Multiple Sclerosis: Results From the Phase 3 EVOLVE-MS-2 Study. 37th Congress of the European Committee for Treatment & Research in Multiple Sclerosis | October 13-15, 2021. Poster: P673
- Phillips JT, et al: Clinical Significance of Gastrointestinal and Flushing Events in Patients with Multiple Sclerosis Treated with Delayed-Release Dimethyl Fumarate Int J MS Care. 2015; 17: 236-243.
- Fox RJ, et al: Placebo-Controlled Phase 3 Study of Oral BG-12 or Glatiramer in Multiple Sclerosis. N Engl J Med. 2012; 367: 1087-1097.
- Gold R, et al: Placebo-Controlled Phase 3 Study of Oral BG-12 for Relapsing Multiple Sclerosis. N Engl J Med. 2012; 367: 1098-1107.
Imprint
Text/Editorial: Leoni Burggraf
Source: 37
th
Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), 13-15.10.2021
This article was written with the financial support of Biogen Switzerland AG, Baar, Switzerland.
© Prime Public Media AG, Zurich 2021
Biogen-144226_12.2021
™
▼This drug is subject to additional observation. For more information, please refer to the Vumerity SmPC
™
at www.swissmedicinfo.ch. Z: Capsules with enteric-coated microtablets of 231mg diroximel fumarate. I: Treatment of patients with relapsing-remitting multiple sclerosis (RRMS) to reduce relapse frequency. D: Initial dose 231mg twice daily, then increase to 462mg (administered as two capsules of 231 mg) twice daily. Oral use. Can be taken with or without meals. Do not crush, split, suck, or chew the capsules. KI: Hypersensitivity to diroximel fumarate, dimethyl fumarate or excipients. Impaired liver function and moderately or severely impaired kidney function. Infection with the HI virus (HIV). Severe active, as well as active chronic infections. Severe gastrointestinal (GI) disorders. Leukopenia < 3.0×10
9
/l, lymphopenia < 0.5×109/l. Progressive multifocal leukoencephalopathy (PML) or suspected PML. Age < 18 years. Start treatment during pregnancy (S). VM: No concomitant treatment with other fumaric acid derivatives. Cases of anaphylaxis have been reported during treatment with dimethyl fumarate. Laboratory: CBC with Diff-BB: Mandatory. before starting treatment, recommended in the first 1.5 years, min. Every 3 months and at least every 6-12 months thereafter and when clinically indicated. In patients with leukopenia < 3.0×10
9
/l or lymphopenia < 0.5×10
9
/l pause therapy. Benefit-risk assessment in patients with lymphocyte counts of ≥ 0.5×10
9
/l and < 0.8×10
9
/l during > 6 months. The median time to normalization of lymphocyte counts after discontinuation of dimethyl fumarate treatment in patients without prolonged severe lymphopenia is estimated to be 4.7 weeks. With dimethyl fumarate and other fumarate-containing drugs, cases of PML have occurred predominantly in patients with lymphopenia (< 0.91×10
9
/l), cases of PML have occurred. At the first PML signs/symptoms, discontinue Vumerity and perform diagnostic testing. Patients should inform their trusted or caregiver(s) about treatment with Vumerity, as they may perceive symptoms that are not noticed by the patient. Serious herpes zoster infections (HZI) can occur at any time under Vumerity. Appropriate action should be taken if HZI is confirmed. Renal/hepatic function: recommended before as well as 6 months after initiation of therapy, then every 6-12 months, and in appropriate clinical settings. Vumerity may cause drug-induced liver injury, including liver enzyme elevation (≥ 3x ULN) and total bilirubin elevation (≥ 2x ULN). Use only with caution in mild renal impairment as well as nephrotoxic comedication. Consider interruption of therapy in case of severe infections. When switching from disease-modifying therapy and/or immunosuppressants (IS) to Vumerity, perform close clinical follow-up regarding opportunistic infections during the first few months of treatment. S: Contraception obligatory in childbearing age. Not recommended during S; if recommended, only if clinical findings mandate and patient benefit outweighs fetal risk. Individual decision whether to discontinue breastfeeding or treatment with Vumerity. The benefits of breastfeeding for the child and the benefits of therapy for the mother must be considered. UW: gastroenteritis, lymphopenia, leukopenia, sensation of burning, flushing, flushing, gastrointestinal disorders (diarrhea, nausea, upper/abdominal pain, vomiting, dyspepsia, gastritis, GI upset), pruritus, rash, erythema, proteinuria, sensation of heat, ketonuria, elevation AST and ALT. IA: Increased risk of infection with concomitant treatment with IS. In case of pretreatment with IS, immunocompetence must be restored before starting therapy. No effect of 325mg ASA on PK profile. List B. The complete technical information is published at www.swissmedicinfo.ch. Biogen Switzerland AG, Neuhofstrasse 30, CH-6340 Baar. Status of information: August 2021. Biogen-129742_09.2021