Determination of EGFR mutational status and EML-ALK translocations in patients with metastatic adenocarcinoma has therapeutic implications and should be requested at diagnosis. Maintenance treatment with pemetrexed in patients with non-squamous cell carcinoma has entered routine clinical practice. A major challenge is secondary resistance to oral EGFR TKIs resp. Crizotinib. Established second-line therapies in patients with tumors without a “genetic target” have limited activity, and predictive actuators would be of considerable importance here.
Worldwide, lung cancer remains the most common malignancy in men and the fourth most common malignancy in women. While the incidence of non-small cell lung cancer (NSCLC) has declined somewhat in men in many countries over the past decade, NSCLC is increasing in women in quite a few industrialized countries, for example from 35 to 39 per 100,000 persons in the United Kingdom between 1993 and 2008. Mortality due to NSCLC in Europe increased by 10% in women between 2007 and 2012 [1]. This is a sad consequence of “emancipated” smoking. Approximately 85-90% of all lung cancers involve the non-small cell variety, and up to two-thirds of all patients are diagnosed at the metastatic stage. For clarity, this article refers to NSCLC and metastatic stage.
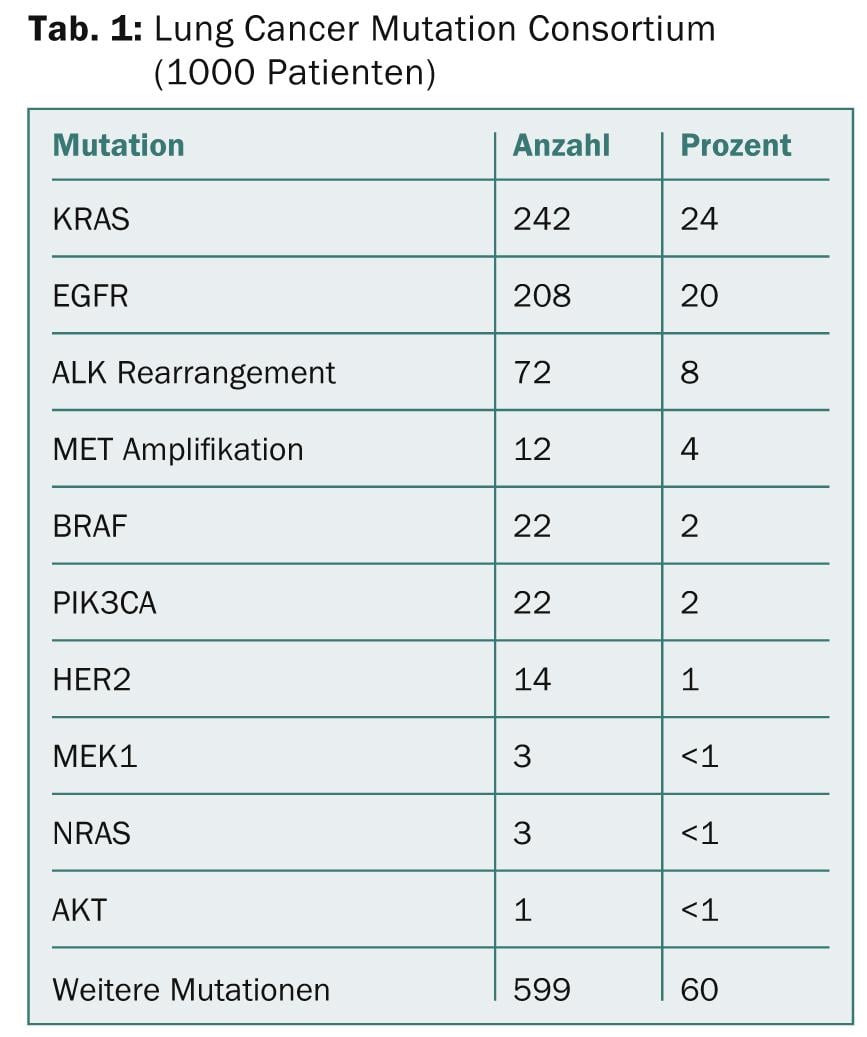
Molecular subclassification of NSCLC led to approval of potent oral oncologics in patients with activating EGFR mutations (10% of Caucasians) (erlotinib, gefitinib, afatinib) resp. EML-ALK translocations (5% of Caucasians) (crizotinib). Additional “driver” mutations in adenocarcinoma (ROS1, RET, HER2, BRAF, KRAS, MET) and squamous cell carcinoma (FGFR1, PIK3CA, DDR2, PTEN) may allow targeted treatments in the future [2] (Table 1).
Diagnostics
For histo- or cytopathological diagnosis in the (frequent) case of limited tumor material from fine needle or punch biopsies, corresponding recommendations were published in 2011 [3]. In any case, a subtyping into adenocarcinoma resp. squamous cell carcinoma should be targeted, and the rate of tumors that cannot be further typed should be limited to <10%. For adenocarcinomas, primary determination of EGFR and EML-ALK mutation status is recommended [4].
Metastatic NSCLC without evidence of activating EGFR mutations.
First-line treatment: First-line treatment in patients without evidence of activating EGFR mutations takes into account the histologic subtype of the disease. Here, patients with adenocarcinomas receive a combination of the antifolate pemetrexed and a platinum salt, either cisplatin in the absence of contraindications, or paraplatin. In patients with squamous cell carcinoma of the lung, pemetrexed is replaced by the nucleoside analog gemzitabine or a taxane. Differentiated treatment by histotype dates back to a randomized trial showing the superiority of cisplatin/pemetrexed over cisplatin/gemzitabine in adenocarcinomas (overall survival 12.6 vs. 10.9 months), and vice versa in squamous cell carcinomas (10.8 vs. 9.4 months) [5].
Regarding the potential addition of the anti-VEGF antibody bevacizumab to standard chemotherapy, a clear “transatlantic divide” is opening up. The American ECOG-4599 trial showed a significant prolongation of overall survival from 10.3 to 12.3 months with the addition of bevacizumab to carboplatin and paclitaxel, with bleeding complications occurring in 4.4% of bevacizumab-treated patients [6]. In the AVAiL randomized European comparative trial, bevacizumab was combined with cisplatin and gemzitabine without resulting in a significant or clinically relevant improvement in survival [7]. Despite these data, approval of bevacizumab in Switzerland was exclusively in combination with cisplatin/gemzitabine, and its use in NSCLC is limited.
Elderly patients and those with a limited performance status: We usually refer to patients with an age of at least 70 years as “elderly”. These patients are more likely to have organ dysfunction and impaired performance status or to be taking potentially interacting medications. Epidemiological trends in Western countries explain the particular importance of this rapidly growing patient group.
One randomized trial addressed the question of optimal first-line treatment in patients aged 70 to 89 years with a performance status up to 2 [8]. This showed that combination chemotherapy with carboplatin and weekly paclitaxel was superior to monotherapy with gemzitabine or vinorelbine, and resulted in an improvement in survival from 6.2 to 10.3 months. Thereby, chemotherapy-related complications were not clustered compared with monotherapy. Thus, overall, we prefer platinum-based combination therapy even in elderly patients, as well as in selected patients with a performance status of 2 and with at least good organ function. Monotherapies are recommended in the case of elderly patients with performance status 2 or substantial comorbidities.
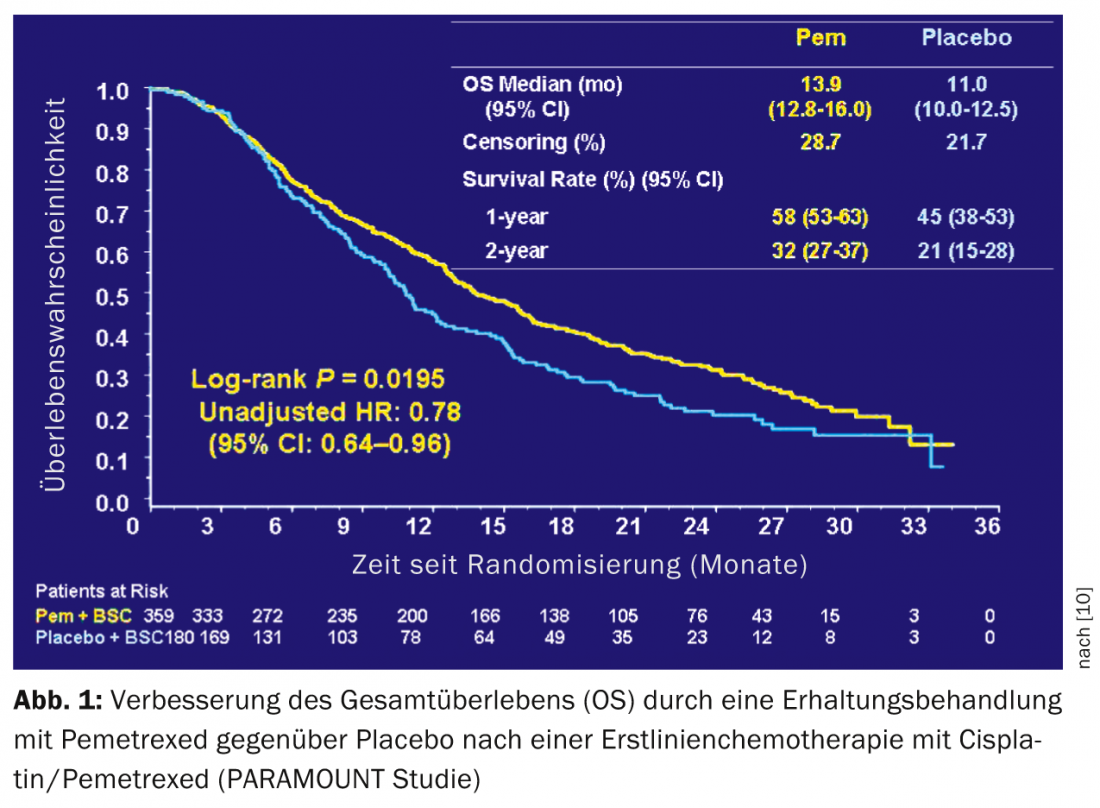
Maintenance therapy: two randomized trials have demonstrated the clinical benefit of maintenance therapy after four cycles of platinum-based chemotherapy [9,10]. SATURN showed a significant but small improvement in survival from 11 to 12 months with maintenance treatment with erlotinib. This benefit was also shown in patients with EGFR wild-type. PARAMOUNT demonstrated an improvement in survival from 11.0 to 13.9 months with maintenance treatment with pemetrexed after four cycles of cisplatin/pemetrexed in patients with non-squamous cell carcinoma of the lung (Fig.1). Maintenance treatment with pemetrexed has become a frequent strategy in this patient group, not least because of the good tolerance of pemetrexed.
Second-line therapy: on average, disease progression occurs in patients with metastatic NSCLC five months after initiation of first-line therapy, and many of these patients are eligible for a second line of therapy. The most commonly used oncology agents are pemetrexed, docetaxel, and the anti-EGFR tyrosine kinase inhibitor erlotinib. While docetaxel and erlotinib have no histologic subtype restrictions, pemetrexed is approved only for predominantly adenocarcinoma differentiation. The TAILOR trial, published in 2013, showed a survival benefit of second-line docetaxel therapy over erlotinib in patients with metastatic NSCLC and EGFR wild-type (8.2 vs. 5.4 months) [11]. However, the survival benefit in the TAILOR trial is borderline statistically significant and the clinical relevance of the results is controversial.
Metastatic NSCLC with activating EGFR mutations.
Searching for activating EGFR mutations in patients with metastatic adenocarcinoma or, in the case of squamous cell carcinoma, in “few smokers” (<15 pack-years) is recommended at diagnosis [4]. In patients with activating EGFR mutations, first-line therapy with an oral anti-EGFR tyrosine kinase inhibitor (erlotinib, gefitinib) should be preferred over chemotherapy. EURTAC demonstrated a substantial improvement in progression-free survival in patients with activating EGFR mutations treated with erlotinib versus platinum-based combination chemotherapy (9.7 and 5.2 months, respectively) [12]. Comparable results were shown in the Asian IPASS (gefitinib) trial [13] and the LUX-Lung-6 (afatinib) trial [14]. Despite the frequent acneiform exanthema during treatment with anti-EGFR tyrosine kinase inhibitors, overall subjective tolerance is very good, and appropriate treatment can also be considered in patients with performance status >2. The irreversible anti-EGFR tyrosine kinase inhibitor afatinib was approved by the EMA in September 2013; Swissmedic approval was granted in January 2014.
Metastatic NSCLC with EML-ALK translocations (ALK+).
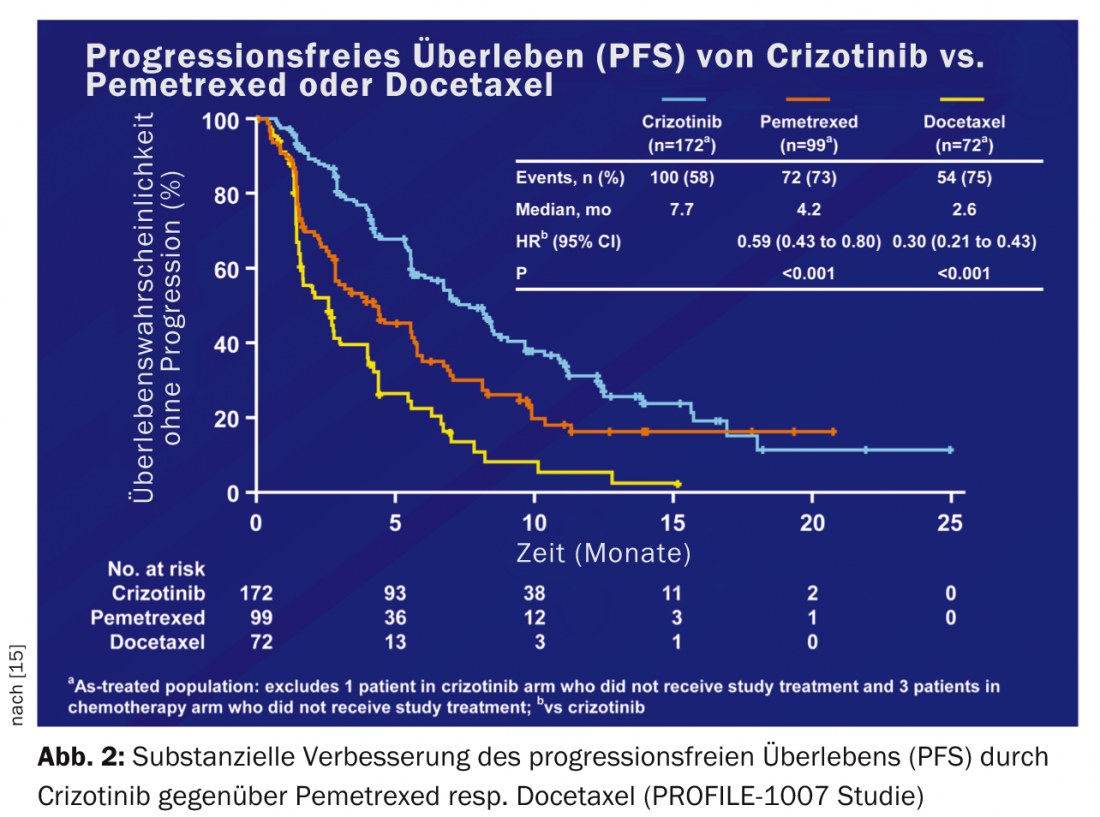
Approximately 5% of Caucasians with NSCLC have an EML-ALK translocation (ALK+). The first randomized data on crizotinib vs. chemotherapy in patients with ALK+ NSCLC after failure of first-line chemotherapy (PROFILE 1007) showed a substantial improvement in progression-free survival from 3.1 to 7.7 months [15] (Fig. 2).
Due to the inevitable development of resistance, intensive efforts are currently underway to develop second-generation ALK inhibitors. The most advanced compounds are the oral ALK/EGFR inhibitor AP26113 and the pure ALK inhibitors LDK378 and CH5424802. In addition to good activity even in resistant ALK+ tumors, these compounds also have the advantage of CNS mobility.
PD Dr. med. et rer. nat. Markus Joerger
Literature:
- Malvezzi M, et al: European cancer mortality predictions for the year 2012. Ann Oncol 2012; 23: 1044-1052.
- Ujhazy P, Herbst R: Personalized therapy. J Thorac Oncol 2012; 7: S401-403.
- Travis WD, et al: International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011; 6: 244-285.
- Peters S, et al: Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23 Suppl 7: vii56- 64.
- Scagliotti GV, et al: Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 2008; 26: 3543-3551.
- Sandler A, et al: Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355: 2542-2550.
- Reck M, et al: Overall survival with cisplatin-gemcitabine and bevacizumab or placebo as first-line therapy for nonsquamous non-small-cell lung cancer: results from a randomised phase III trial (AVAiL). Ann Oncol 2010; 21: 1804-1809.
- Quoix E, et al: Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non-small-cell lung cancer: IFCT-0501 randomised, phase 3 trial. Lancet 2011; 378: 1079-1088.
- Cappuzzo F, et al: Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol 2010; 11: 521-529.
- Paz-Ares LG, et al: PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol 2013; 31: 2895-2902.
- Garassino MC, et al: Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013; 14: 981-988.
- Rosell R, et al: Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239-246.
- Mok TS, et al: Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947-957.
- Wu YL, et al: Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213-222.
- Shaw AT, et al: Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013; 368: 2385-2394.
InFo ONCOLOGY & HEMATOLOGY 2014; 2(4): 10-12.