GLP-1 receptor agonists not only improve glycemic control but also support weight loss and have also demonstrated additional cardio- and neprotective benefits. Dulaglutide (s.c.) has been successfully used for the treatment of diabetes mellitus for several years. In a stratified subgroup analysis based on data from the AWARD-11 study, treatment with dulaglutide at doses of 3 mg and 4.5 mg resulted in greater weight loss in type 2 diabetics with BMI ≥25 kg/m2 compared with dulaglutide 1.5 mg.
Substances from the GLP-1 receptor agonists group are based on the natural glucagon-like peptide-1 (GLP-1) and belong to the incretin mimetics. GLP-1 is secreted in response to food intake and is involved in the control of glucose metabolism by driving the release of insulin from the pancreas and inhibiting the hormone glucagon, an antagonist of insulin. The promotion of weight loss by GLP-1 receptor agonists (GLP-1-RA) is particularly due to an enhancement of the feeling of satiety and a slowing of gastric emptying [1]. Therefore, the use of representatives of this class of active ingredients is particularly suitable for patients with morbidly increased obesity.
Post-hoc analysis of the AWARD-11 study.
For subcutaneously injected dulaglutide (Trulicity®), the AWARD-11 study (n=1842) demonstrated that in type 2 diabetics in whom metformin was insufficiently effective, dulaglutide at doses of 3 mg and 4 mg (both 1x/week) improved HbA1c levels and body weight compared with dulaglutide 1.5 mg (1×/week) [2]. At week 52, weight loss in patients receiving dulaglutide 3.0 mg and 4.5 mg was 4.3 kg and 5.0 kg, respectively, compared with only 3.5 kg in the 1.5-mg dose [2].
The aim of a post-hoc analysis was to evaluate the effect of dulaglutide on body weight in a subgroup of patients with clinically relevant elevated BMI at baseline [3]. The results were presented by Prof. Enzo Bonora, MD, Verona (I), at this year’s EASD Annual Meeting [4]. Study participants were included with HbA1c values of 7.5-11% and a BMI ≥25 kg/m2. The 1842 patients were randomized to the dulaglutide (s.c.) 1.5 mg or 3 mg or 4.5 mg treatment arms. The total treatment duration was 52 weeks, and the primary efficacy endpoint was collected at week 36. Patients were stratified into four subgroups with respect to BMI (review 1) [4].

Type 2 diabetics with clinically relevant BMI in focus
It was found that patients in whom the dose of dulaglutide was escalated to 3.0 mg or 4.5 mg had an additional weight reduction at week 36 and 52, respectively, regardless of baseline BMI [3,4]. Absolute weight loss was higher in patients with higher BMI, but mean percent weight loss proved similar in all four BMI subgroups. At week 52, nearly 50% of patients on dulaglutide 4.5 mg achieved the ≥5% weight loss mark recommended by current guidelines, regardless of baseline BMI (Fig. 1). The incidence of common adverse events was comparable across all subgroups of BMI categories.
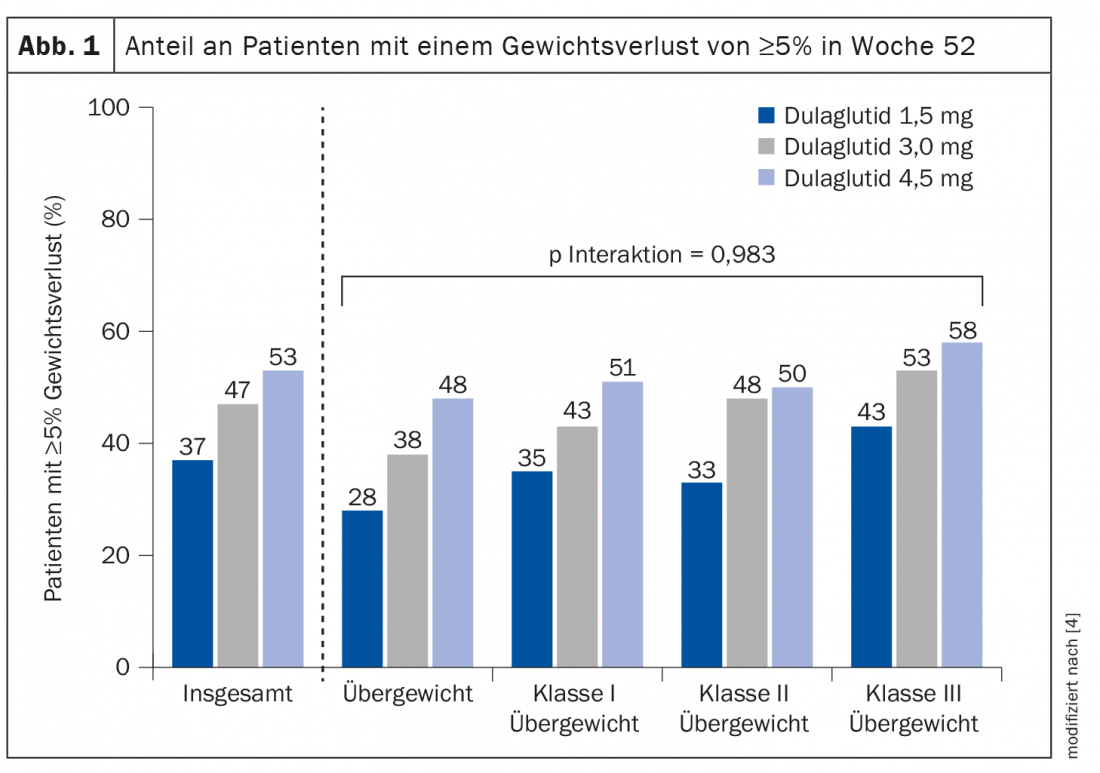
In summary, all four BMI categories (25- ≥40 kg/m2) benefited from an additional benefit in terms of weight reduction with dose escalation of dulaglutide to 3.0 mg or 4.5 mg.
Congress: EASD Annual Meeting 2021
Literature:
- Blundell J, et al: Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes, Obesity and Metabolism 2017; 19 (9): 1242-1251.
- Frias JP, et al: Efficacy and Safety of Dulaglutide 3.0 mg and 4.5 mg Versus Dulaglutide 1.5 mg in Metformin-Treated Patients With Type 2 Diabetes in a Randomized Controlled Trial (AWARD-11). Diabetes Care 2021; 44(3): 765-773.
- Bonora E, et al: Higher doses of dulaglutide induce weight loss in patients with type 2 diabetes regardless of baseline BMI: post hoc analysis of AWARD-11. 57th EASD Annual Meeting of the European Association for the Study of Diabetes. Diabetologia 2021; 64: 1-380.
- Bonora E: Higher doses of dulaglutide induce weight loss in patients with type 2 diabetes regardless of baseline BMI: post-hoc analyses of AWARD-11. SO 28 GLP-1-receptor agonists and weight loss. EASD Annual Meeting, Sept. 29, 2021.
HAUSARZT PRAXIS 2021; 16(11): 34 (published 11/14-21, ahead of print).











