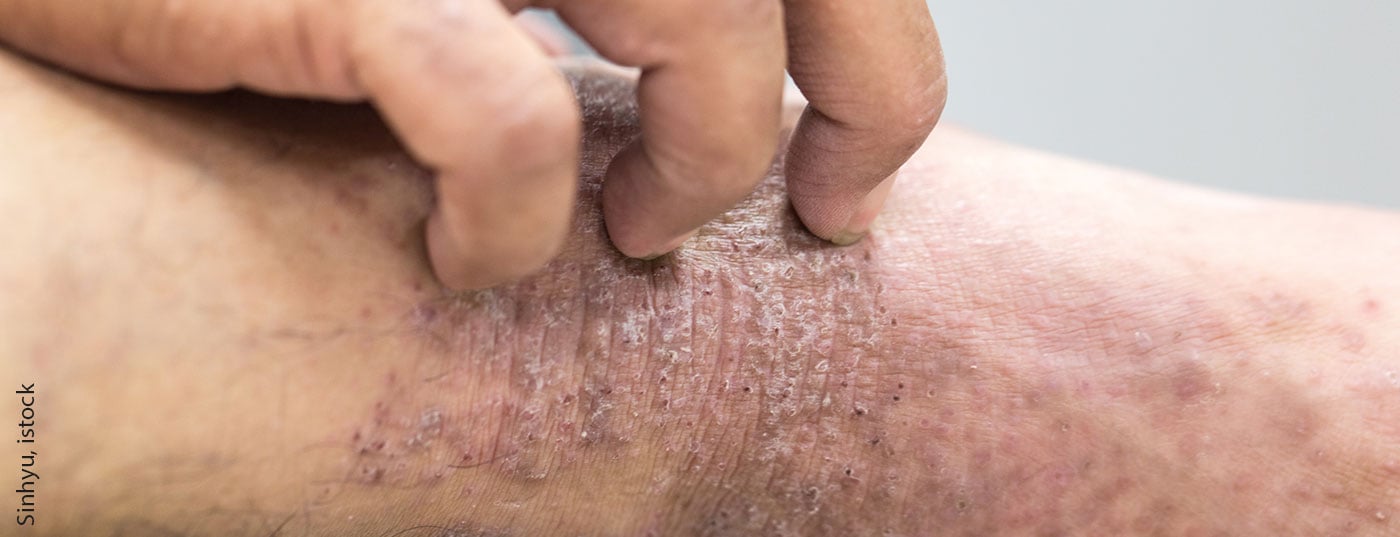
Especially in adolescents, moderate to severe atopic dermatitis is associated with a high degree of suffering. In the pivotal study, dupilumab resulted in rapid and sustained relief of skin lesions and itching. Health-related quality of life also improved significantly. Dupilumab is currently the only biologic approved in Switzerland for this patient group.
Swissmedic has extended the November 2020 marketing authorization for Dupixent® (dupilumab) for the treatment of moderate-to-severe atopic dermatitis to adolescents aged 12 to 17 years with moderate-to-severe atopic dermatitis when therapy with prescription topical medications does not provide adequate disease control or is not recommended. [1]. There is a need for effective treatment options with few side effects, particularly for adolescent patients with moderate or severe atopic dermatitis. Long-term therapy with glucocorticoids has limited efficacy and is associated with side effects such as skin atrophy. Inadequately treated atopic dermatitis can have negative effects on the physical, psychological and psychosocial levels. Anxiety and sleep disorders, as well as depression and social exclusion, are clustered among those affected [2]. “Moderate to severe atopic dermatitis can impact an adolescent’s life in many ways, affecting both their physical and psychological well-being,” said Lisa Weibel, MD, Head of the Department of Pediatric Dermatology, University Children’s Hospital Zurich [3].
Fast and lasting improvement of itching and skin lesions
The Swissmedic approval of dupilumab for adolescents is based on clinical data from the LIBERTY AD program. The efficacy and safety of monotherapy with the IL4/IL13 inhibitor at a dose of 200 mg or 300 mg every two weeks was assessed in a multicenter, randomized, double-blind, placebo-controlled study in 251 patients 12 to 17 years of age with moderate to severe atopic dermatitis who had an inadequate response to topical therapy [4]. Overview of important results after the 16-week treatment period:
- EASI-75: 42% of patients treated with dupilumab compared with 8% of patients treated with placebo achieved at least 75% improvement in skin lesions (EASI-75)* [4].
- IGA 0/1: 24% of patients achieved IGA** 0 or 1 on dupilumab compared with 2% on placebo [4].
- EASI improvement: patients treated with dupilumab showed a mean improvement in EASI of 66% from baseline, compared with 24% in patients treated with placebo [4].
- Reduction of pruritus: 37% of patients achieved a clinically relevant improvement of at least four points on the Peak Pruritus Numerical Rating Scale (NRS) with dupilumab, compared to 5% with placebo [5].
- Quality of life improvement: 61% of patients treated with dupilumab compared with 20% of those treated with placebo achieved a clinically meaningful improvement in quality of life of at least 6 points on the Children’s Dermatology Life Quality Index (CDLQI) [5].
- Patient Oriented Eczema Measure (POEM): With dupilumab, 63% of patients had at least a 6-point improvement in disease severity as measured by the Patient Oriented Eczema Measure (POEM). This assesses various aspects, including sleep, among others. Under placebo, this proportion was 10% [5].
* EASI = Eczema Area and Severity Index
** IGA = Investigator’s Global Assessment
|
High “Burden of Disease” in adolescents with atopic dermatitis. “Adolescents with inadequately controlled moderate to severe atopic dermatitis face several problems that can have lasting effects on their future lives. The physical symptoms and psychological impact of moderate to severe atopic dermatitis can prevent adolescents from fully participating in everyday life, such as school, sports, and other leisure activities,” says PD Lisa Weibel, MD, Head of the Department of Pediatric Dermatology, University Children’s Hospital Zurich [3]. “We know from the Phase III study that dupilumab significantly reduces itching and skin lesions. It also improves health-related quality of life in adolescents at this important stage of their lives.” |
No determination of laboratory values required
In terms of tolerability, there was no difference from previous experience in adults: Dupilumab treatment was most frequently associated with injection site reactions and conjunctivitis [1,4]. Treatment with dupilumab does not require determination of laboratory values at baseline or during treatment [1]. Dupixent® (dupilumab) is available as a pre-filled syringe in two dosages (200 mg and 300 mg). In adolescents with moderate to severe atopic dermatitis, the dosage depends on body weight. Dupilumab is injected subcutaneously, at a starting dose of 400 mg (<60 kg) and 600 mg (≥60 kg), respectively, followed by 200 mg and 300 mg every two weeks. The injection can be performed in a clinic/practice or, after appropriate instruction by healthcare professionals, can be performed at home by the patient. In adolescents, it is recommended that dupilumab be administered under adult supervision [1].
Source: Sanofi-Aventis
Literature:
- DUPIXENT®: SmPC, www.swissmedicinfo.ch, (last accessed 19.03.2021).
- Langan SM, et al: Atopic dermatitis. Lancet 2020; 396(10247): 345-360.
- “Swissmedic approves the use of DUPIXENT® (dupilumab) for the treatment of moderate to severe atopic dermatitis now also for adolescents aged 12 years and older,” Sanofi-Aventis (Switzerland) Ltd, Feb. 24, 2021.
- Simpson EL: JAMA Dermatol 2020; 156; 44-56.
- Paller AS, et al: Am J Clin Dermatol. 2020; 21(1): 119-131.
- Gandhi NA, et al: Nat Rev Drug Discov 2016; 15: 35-50.
HAUSARZT PRAXIS 2021; 16(5): 28