“Shaping the future together” was the motto of this year’s urology congress. Because health care must evolve. It is not only colleagues in the field of urology who are in demand. Interdisciplinary management is indicated to defy the challenges of the diseases and to focus more on individualization, especially in the field of uro-oncology.
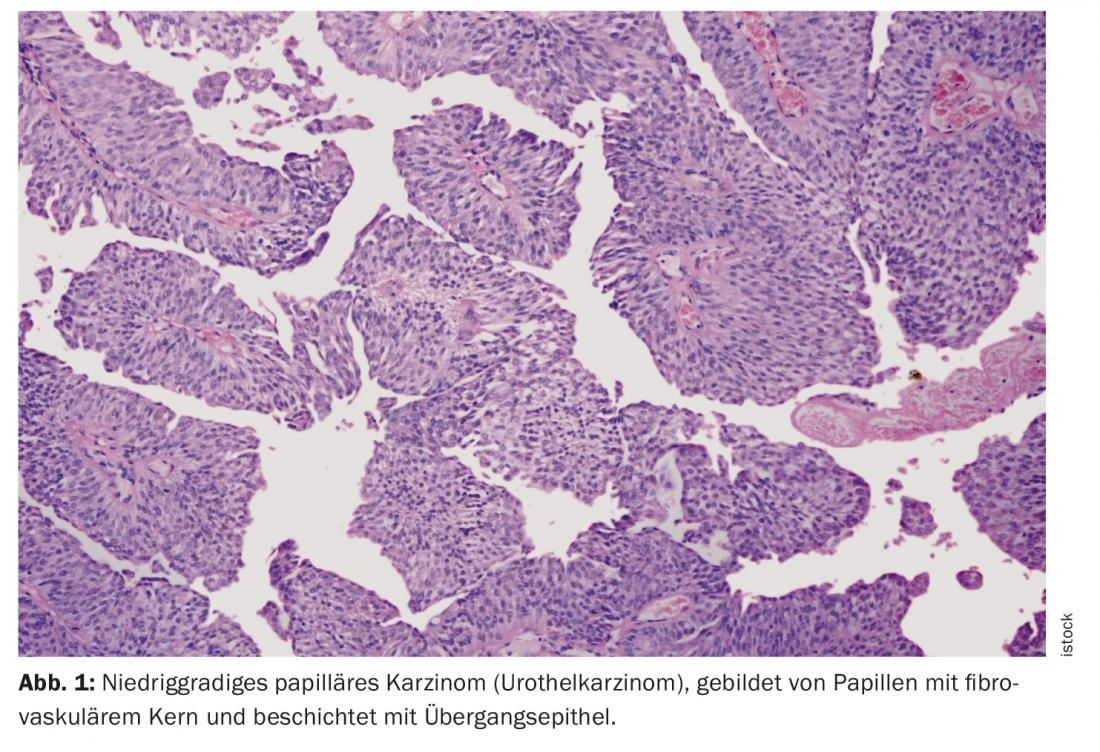
One of the DGU’s topics dealt with the ever-increasing individualization of patient therapy, especially in the field of uro-oncology. Here, joint action by interdisciplinary teams of urologists, oncologists, radiation therapists, radiologists, pathologists, and possibly other disciplines is required to optimize patient care. Advanced urothelial carcinoma (UC), in particular, continues to pose major challenges to practitioners. UC is a form of bladder cancer. The carcinoma originates in the urothelium, the epithelial layer lining the urinary tract. More than 90% of all urothelial carcinomas involve the urinary bladder. Furthermore, it can occur in the renal pelvis, ureter or urethra.
The main risk factor for the development of bladder cancer is active and passive smoking [1]. The risk of disease increases with the duration and amount of exposure. In addition, aromatic amines have a carcinogenic effect [2]. Occupational exposure used to play a relevant role, especially in the chemical industry, construction and health services. Today, corresponding substances have been largely eliminated from everyday working life. However, because the latency period between exposure and diagnosis averages about 38 years, there are still cases with this etiology today. In addition, local radiotherapy, the antidiabetic drug pioglitazone, and some air and water pollutants may also increase the risk of disease [1].
Treatment of urothelial carcinoma
Superficially growing tumors can be resected transurethrally (TUR), sometimes requiring resection to remove all tumor tissue [2]. Depending on tumor classification and risk classification for aggressive growth, additional instillations with Bacillus Calmette-Guérin (BCG) or chemotherapeutic agents are recommended for recurrence prophylaxis. Invasively growing tumors should undergo cystectomy. If necessary, additional radiation and/or neoadjuvant or adjuvant chemotherapy is given [2]. In the metastatic stage, cystectomy is usually not performed – standard first-line treatment is then cisplatin-containing chemotherapy. But platinum-based chemotherapies often reach their limits. On the one hand, 30 to 50 percent of patients are not suitable for this. Second, despite high response rates, they usually achieve only limited overall survival (OS). With cisplatin/gemcitabine, the median OS is 7.7 months and with carboplatin/gemcitabine it is 5.8 months [3–5]. Also the option with checkpoint inhibitors does not bring the desired result. The median OS after immunotherapy is 15.9 months [2,6]. Accordingly, immunotherapy is usually ineffective in the first-line setting, and it is usually too late for affected individuals to receive a second-line setting in which immunotherapy might be used. Only about one-third of patients receive second-line treatment [7–9]. Effective maintenance therapy is therefore indicated.
First-line maintenance therapy with the standard of care
If the cancer has not progressed after platinum-based chemotherapy, the PD-L1 inhibitor avelumab is available for first-line maintenance therapy. In combination with best supportive care (BSC), the treatment leads to prolonged OS and progression-free survival (PFS) – even in the long term, as demonstrated by results of a recent long-term follow-up of the JAVELIN Bladder 100 trial [10,11]. The multicenter, multinational, randomized, open-label phase III study enrolled 700 patients with unresectable locally advanced or metastatic urothelial carcinoma after platinum-containing chemotherapy. Randomized in a 1:1 ratio, they received either avelumab 10 mg/kg IV every two weeks and BSC or BSC alone. After two years, OS rates were 49.8% in the combination group versus 38.4% in the BSC alone group. The 2-year PFS rates were 23.4% vs. 7.1%, respectively. The response of the patients to the previous chemotherapy had no influence on the effect of the maintenance therapy. The most common treatment-related adverse events (TEAEs) were urinary tract infections, diarrhea, and joint pain. However, these led to treatment discontinuation in only a small number of those affected. Confirmation of the acceptable safety profile as well as the efficacy of avelumab treatment could be obtained by initial findings from daily practice [12]. After a median observation period of 13.5 months, early results from the AVENANCE non-interventional study demonstrate clinical activity and safety even in a heterogeneous patient population. OS rates of 66.9% and PFS rates of 36.9% were observed.
Prostate carcinoma – prognosis and prediction
According to the National Comprehensive Cancer Network (NCCN) clinical practice guidelines, patients with localized prostate cancer can be categorized as low-risk, intermediate-risk, or high-risk based on their clinical outcomes. Intermediate and high-risk patients with localized prostate cancer are often treated with definitive external beam radiation therapy (EBRT) in combination with androgen deprivation therapy (ADT). Numerous large phase III cohort studies have shown that the combination of ADT and EBRT can significantly improve prostate cancer-specific mortality (PCSM), distant metastasis (DM), and biochemical recurrence (BR) rates. Measurement of serum levels of prostate specific antigen (PSA) is a valuable biochemical method for prostate cancer screening, monitoring treatment response, and detecting disease recurrence. Prostate specific antigen (nPSA) nadir levels have been shown to predict BR, DM, cause-specific mortality (CSM), and all-cause mortality (OM) after radiotherapy (RT). In addition, there is increasing evidence that time-limited PSA measurements in patients undergoing definitive EBRT are independent early predictors of BR and DM. However, the prognostic value of nPSA in prostate cancer patients treated concurrently with ADT and EBRT remains unclear. The objective of one study was to determine whether an nPSA threshold of 0.06 ng/ml 12 months after treatment can serve as an early predictor of biochemical recurrence-free survival (BRFS), PCSM, and overall survival (OS) in prostate cancer patients treated with concurrent ADT and EBRT.
Retrospectively, clinical data from 338 patients with intermediate- and high-risk prostate cancer were evaluated. The median radiation dose was 76 Gy, the median baseline PSA level was 17 ng/ml (range 1-228 ng/ml), and the median duration of ADT was 24 months (range 6-167 months). The median PSA level 1 month after EBRT was 0.06 ng/ml (range 0-25.6 ng/ml). The median follow-up time was 5 years. Multivariate analysis revealed that nPSA was an independent and significant factor associated with OS, PCSM, and BRFS. Furthermore, time to nPSA12 was an independent predictor of PCSM and BRFS. Pelvic irradiation was also significantly associated with worse OS and PCSM. In addition, age (≤70 or >70 years) and duration of hormone therapy (6 months, 1-3 years, or >3 years) were significantly associated with OS and PCSM, respectively. At high risk, nPSA and nPSA12 were independent predictors of BRFS. An nPSA12 level of >0.06 ng/ml may independently predict worse PCSM and BRFS in patients with intermediate- and high-risk prostate cancer undergoing EBRT and ADT. At high risk, nPSA >0.06 ng/mL and nPSA12 >0.06 ng/mL may independently predict worse BRFS [13].
Kidney cancer – survival benefits from immune checkpoint inhibition
Kidney cancer is a common malignancy with more than 430,000 new cases worldwide in 2020 and approximately 180,000 deaths. Renal cell carcinoma (RCC) accounts for the majority of renal cancers (90-95%), with clear cell RCC being the most common histologic subtype. Approximately 30% of RCC cases are diagnosed at an advanced or metastatic stage and nearly 80% of these patients have intermediate or poor risk according to the International Metastatic renal cell carcinoma Database Consortium (IMDC) criteria. Renal cell carcinoma is characterized by inactivation of the von Hippel-Lindau tumor suppressor gene, leading to high expression of proangiogenic vascular endothelial growth factor (VEGF).
Until recently, first-line therapy of advanced RCC consisted mainly of the use of tyrosine kinase inhibitors (TKIs). These target the vascular endothelial growth factor (VEGF) receptor, among others. Exploration of novel therapeutic regimens focused on the use of multiple TKIs in combination with monoclonal antibodies that directly inhibit VEGF and act as inhibitors of the Mammalian Target of Rapamycin. VEGF and VEGF receptor inhibitors are thought to have immunomodulatory effects and also promote immune cell infiltration due to their effect on tumor vessels. While vascular endothelial growth factor-targeted single-agent therapy has been a mainstay of treatment, data from multiple phase III trials evaluating combinations of immune checkpoint inhibitors (ICIs) as first-line treatment have shown significant survival benefit. In a review, six phase III trials showed significant benefits for ICI combinations compared with sunitinib. Nivolumab plus ipilimumab significantly improved overall survival (median 47.0 versus 26.6 months) and progression-free survival (median 11.6 versus 8.3 months) in intermediate- and poor-risk International Metastatic Renal Cell Carcinoma Database Consortium patients. Overall survival was also significantly improved with combinations of ICI and tyrosine kinase inhibitors regardless of risk, including pembrolizumab plus axitinib or lenvatinib and nivolumab plus cabozantinib. No new safety signals were detected [14].
Congress: 74th Congress of the German Society of Urology (DGU)
Literature:
- Robert Koch Institute, Cancer in Germany for 2017/2018. 13th edition, chapter 3.25 Urinary bladder.
- S3 Guideline Early Detection, Diagnosis, Therapy, and Follow-up of Urinary Bladder Cancer 2020. Available online at www.awmf.org/leitlinien/detail/ll/032-038OL.html.
- Von der Maasse H, et al: J Clin Oncol 2000; 18(17): 3068-3077.
- Von der Maasse H, et al: J Clin Oncol 2005; 23(21): 4502-4608.
- De Santis M et al. J Clin Oncol 2012; 30(2): 191-199.
- DGHO Guideline Bladder Carcinoma (Urothelial Carcinoma). Available online at www.onkopedia.com/de/onkopedia/guidelines/blasenkarzinom-urothelkarzinom/@@guideline/html/index.html.
- Aly A, et al: J Med Econ 2019; 22(7): 662-670.
- Cheeseman S, et al: Front Oncol 2020; 10: 167.
- Niegisch G, et al: J Cancer 2018; 9(8): 1337-1348.
- Powles T, et al: Abstract 487. Presented at ASCO GU 2022.
- Powels T, et al: N Engl J Med 2020; 383: 1218-1230.
- Barthělěmy P, et al: Poster 1757P. Presented at ESMO Congress 2022.
- Cetin IA, Akay SU, Sengoz M: Prostate-specific antigen nadir within 1 year of radiotherapy combined with hormone therapy predicts cancer-specific mortality and biochemical recurrence-free survival in prostate cancer patients. BMC Urol 2022; 22(1): 182.
- Lalani AKA, Heng DYC, Basappa NS, et al: Evolving landscape of first-line combination therapy in advanced renal cancer: a systematic review. Ther Adv Med Oncol 2022; 14: 17588359221108685.