By definition, vitamins are substances that the human organism cannot produce itself, but which it needs to live and which must therefore be supplied. However, the precursors of vitamin D are produced by the body itself. This “pro-hormone”, as it should actually be called, must then be supplemented by sunlight. Bone pain, muscle weakness, and various nonspecific symptoms may indicate vitamin D deficiency. However, it is also frequently asymptomatic. Most of the time, therefore, an undersupply is missed. In the summer of 2012, the Federal Office of Public Health formulated recommendations for the year-round supply of vitamin D3 for the healthy population. In the following article, the practical procedure of supplementation is explained and tricky situations are described.
The fat-soluble vitamin D3 is formed to 80% in the skin by sun radiation. About 20% is supplied through the diet. Fatty sea fish (mackerel, eel, salmon) has the highest vitamin D content. It is also found in small amounts in milk, egg yolk and mushrooms [1].
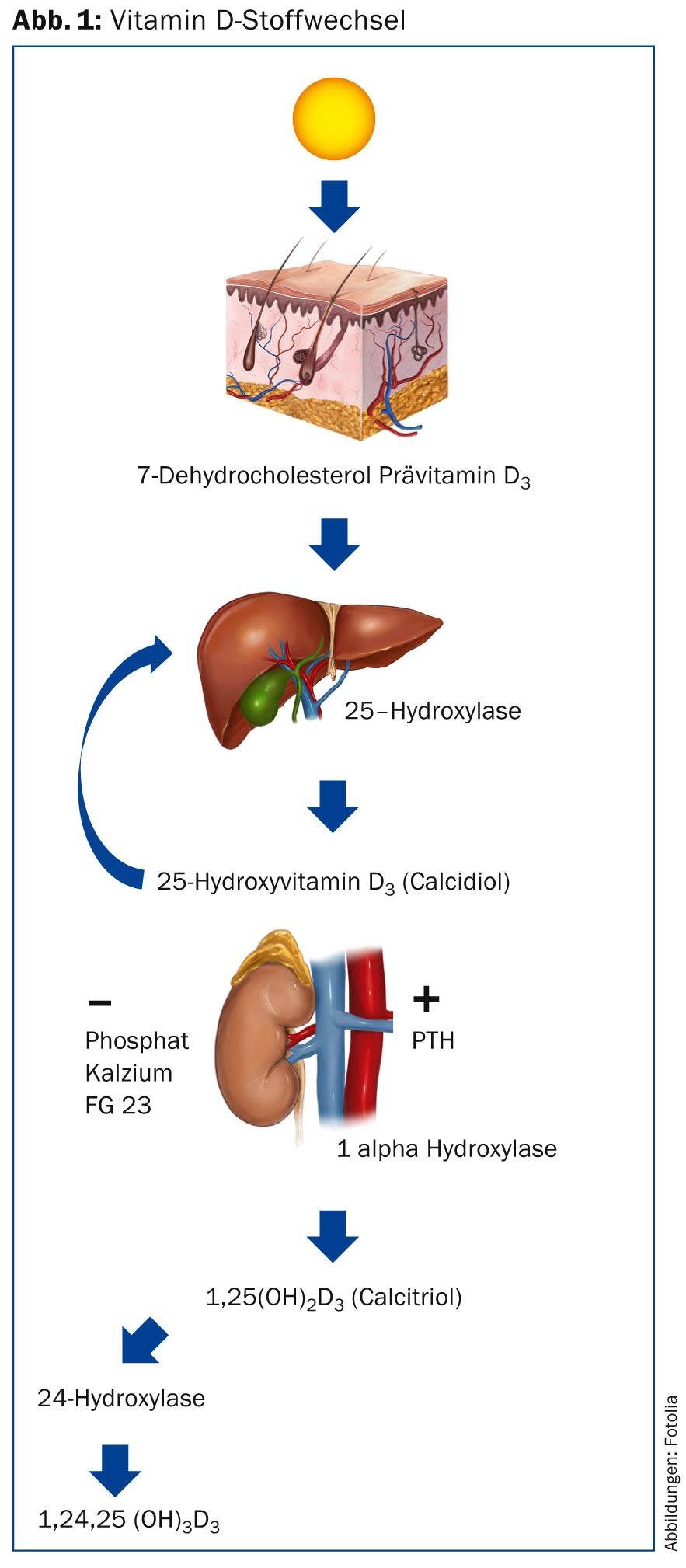
In the skin, vitamin D3 (cholecalciferol) is formed from 7-dehydrocholesterol under the influence of UVB radiation after an intermediate step; a wavelength of 290-315 nm is required for this. This is coupled to the vitamin D-binding protein and transported via the bloodstream to the liver, where it is hydroxylated at position 25. 25-hydroxyvitamin D3 (25[OH]D3, calcidiol), the storage form of vitamin D3, is formed. Blood level measurements are also taken by this person to check a patient’s vitamin D supply. In a further step, hydroxylation occurs in the kidney by 1α-hydroxylase at position 1, resulting in 1,25-dihydroxyvitamin D3 (1,25[OH]2D3, calcitriol), the active vitamin D3. In the absence of stimulatory signals for enzyme activation, hydroxylation at position 24 leads to inactivation. Cholecalciferol is related to steroid hormones and thus to cholesterol. Vitamin D2, ergocalciferol, has an additional methyl group at position 24 and is found in plant products such as mushrooms.
Clothes, sunblocks with UVB filters, clouds and a flat sunlight incidence, as it occurs diurnally in the morning and evening hours and seasonally in the winter half of the year, prevent the formation of vitamin D in the skin. Dark skin color and older age with decreasing skin thickness and decreased levels of 7-dehydrocholesterol are other factors that lead to up to fourfold decreased vitamin D synthesis in the skin at the same UV radiation dose [2]. Nevertheless, relevant increases in 25(OH)D3 levels can be realized even in older individuals with regular sun exposure [3]. When spending time in a solarium, it depends on the wavelength of the UV light used: Since modern solariums mainly use long-wave UVA radiation, no cholecalciferol is built up. UVA-only irradiation actually degrades vitamin D instead of building it up [4].
While the production of calcidiol in the liver – depending on the supply of cholecalciferol – is more or less continuous, that of calcitriol, which takes place mainly in the kidney by 1α-hydroxylase, is tightly regulated (Fig. 1) [1]. High levels of calcium, phosphate and FGF-23 inhibit production, while parathyroid hormone (PTH), calcitonin and lowered phosphate levels increase it. In contrast, the degradation of calcitriol is less tightly regulated and is tied to the activity of 24-hydroxylase in particular, which is activated by highlevels of1,25(OH)2D3 and converts it to the biologically inactive calcitriolic acid via a series of oxidation events at positions 23 and 24. Thus, with the administration of calcitriol, unlike cholecalciferol, there is a risk of hypercalcemia. Consequently, there is a narrow therapeutic application possibility.
Physiologically, vitamin D serves to mineralize the osteoid in bone. If it is missing, rickets occurs in children and osteomalacia in adults. Vitamin D and PTH are the crucial mediators for a balanced calcium and phosphate balance in the body. Vitamin D promotes intestinal absorption of calcium and phosphate and renal-tubular reabsorption of filtered calcium. Vitamin D receptors are found in almost all body tissues! Vitamin D controls more than 200 genes responsible for cell proliferation, differentiation and death. Furthermore, vitamin D exhibits immunomodulatory properties [1].
Vitamin D deficiency clinic
Vitamin D deficiency is often asymptomatic, so it is easy to miss an undersupply. Bone pain and muscle weakness may indicate vitamin D deficiency. A whole range of non-specific symptoms may be associated with vitamin D deficiency: sleep disturbances, fatigue, (winter) depression, freezing, muscle weakness, cramps or twitching, dizziness, blackness before the eyes or nausea, pain in the head, trunk or limbs, susceptibility to infections, and allergies [5].
Table 1 summarizes risk factors for developing vitamin D deficiency.

Table 2 lists vulnerable patient groups that may particularly benefit from vitamin D supplements. At this point, a word on the difference between substitution and supplementation: in menopause, we speak of hormone substitution, and in heroin addicts, opiate substitution. With vitamin D, on the other hand, we give natural-identical cholecalciferol to supplement the body’s own production. Therefore, it is a supplement and not a substitute.
Measured values – measurement problems
What serum levels of 25(OH)D3 are observed in our country? In a study conducted by the Risch Laboratory and the University of Liechtenstein, the vitamin D levels of individuals over 60 years of age were measured [6]. An age-related decrease in levels was observed. The same study showed seasonal variation: the lowest values were observed in winter and spring, and the highest in summer and fall, although the absolute seasonal variation in mean values was small, about 10 nmol/l. Another study from Lucerne with GP patients also showed a slight seasonal effect [7]. It was interesting to note that the males had a higher amplitude of variation with 11 nmol/l than the females with 4 nmol/l. Could it be that the storage of cholecalciferol in the sex-specific increased fat content of the body has a balancing effect here? Is the higher summer score among men due to different leisure and work behaviors and less consistent sunscreen practices? In a separate study, not yet published, we measured vitamin D in 42 nursing home patients in mid-summer. 68% of subjects had 25(OH)D3 levels below 50 nmol/l, indicating vitamin D deficiency.
It should be noted that the measurement of vitamin D levels is not trivial [8]. The most accurate (and also the most expensive and cumbersome) is high-performance liquid chromatography (HPLC). However, immunological methods can also produce reliable results. Some assays do not distinguish between vitamin D2 (ergocalciferol) and vitamin D3. This is significant when supplemented with vitamin D2, as is the case in the US. Furthermore, the question arises whether inactive metabolites are also measured. Also, especially in the lower measuring range below 25 nmol/l, a coefficient of variation above 10% is to be expected. In case of doubt, it is worthwhile to determine a second sample in another laboratory using a different measurement method and to determine the PTH as well, since elevated values are often found in cases of pronounced vitamin D deficiency. Correct preanalytics must be observed: Since vitamin D is fat-soluble, blood sampling after a fat-containing meal may show distorted values; fasting determination is best.
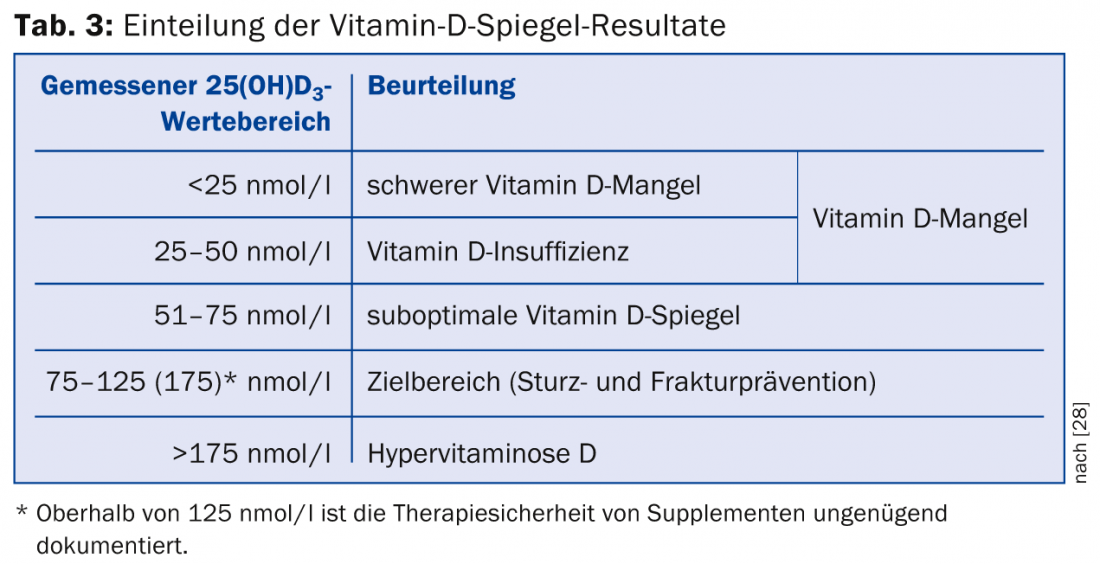
The 25(OH)D3 is measured as a decisive parameter for estimating the vitamin supply. The analysis costs 42 tax points at 90 Rp. In addition, the processing fee of 24 tax points will be charged. So together about 60.- Fr. Values below 25 nmol/l indicate a severe deficiency, values up to 50 nmol/l an “insufficiency”, i.e. an insufficient effect under certain circumstances (Tab. 3).
The determination of 1,25(OH)2D3 is more expensive at 85 tax points and should be left to the specialist. The value does not reflect vitamin D supply; paradoxically, it may be normal or even elevated despite vitamin D deficiency.
The chicken and egg question
An association between low serum vitamin D levels and disease incidence or progression has been observed in many diseases (Table 4) [5].
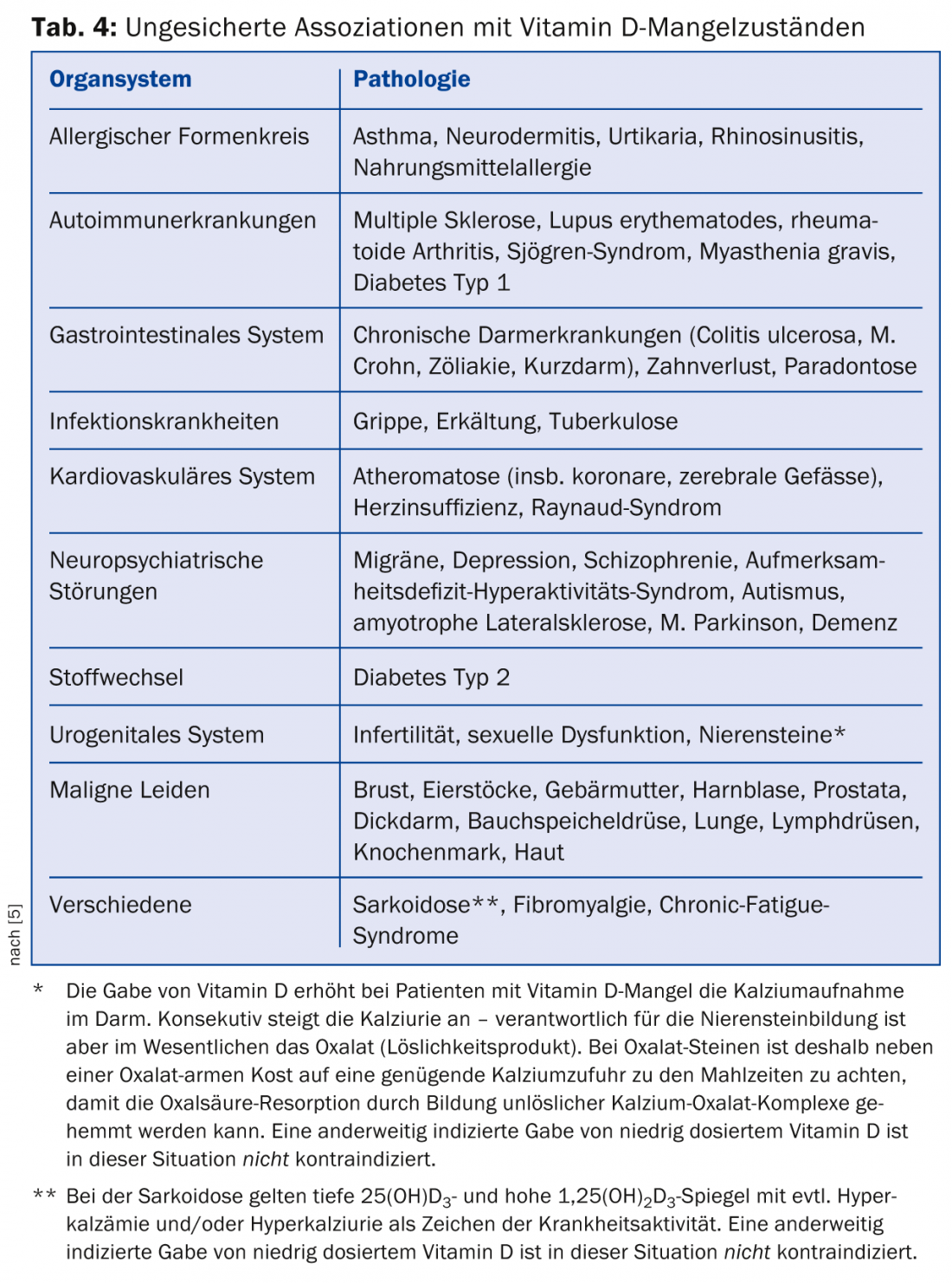
However, we do not know whether the deep mirrors
- are causally linked to the development or deterioration of the respective health disorder,
- are generated by itself, i.e. are disease markers in the narrower sense of the term
- originate from an unknown common cause,
- or caused by the disease indirectly through a change in life circumstances.
Example: patients with Parkinson’s disease regularly have low vitamin D levels [9]. However, they tend to be more immobile than healthy individuals and have less exposure to sunlight, which would support the latter hypothesis. However, in a cohort study, it was observed that Parkinson’s patients had lower vitamin D levels up to 20 years before disease diagnosis, which may indicate that the first three hypotheses are correct.
The human mind tends to want to divide the world into causalities of cause and effect (“MS patients have low vitamin D levels, so vitamin D deficiency worsens the autoimmune brain process, and thus vitamin D supplements should inhibit disease progression”). And even if we can establish a causal relationship between vitamin D administration and well-being resp. of preventing disease symptoms as a given, it is then still not clear whether the dose-response curve of supplements is linear or, as is so often the case, U-shaped (“If a little vitamin D helps a little, a lot of vitamin D helps a lot”). Only intervention studies can provide information here.
Recent research from observational studies, for example, provides evidence that vitamin D deficiency is associated with an increased risk of cardiovascular disease, certain cancers, and type 1 diabetes mellitus. However, this does not yet prove causality. Whether supplementation can delay the onset or progression of these diseases has not yet been proven, so no clear recommendations can be made.
Intervention studies
Most intervention studies examined bone density and fracture and fall risk. In addition to preventing rickets, which of course was not documented in placebo-controlled studies of today’s design at the time, a large meta-analysis provided evidence that at least 800 E of vitamin D per day prevented falls and femoral neck fractures by about 30%, especially when serum levels of 75 nmol/l or more were achieved. In another meta-analysis, vitamin D supplements demonstrated a significant gain in mineralized bone at the femoral neck compared with placebo [10].
Recommendations
In the summer of 2012, the Federal Office of Public Health formulated the following recommendations for the year-round supply of vitamin D3 for the healthy population [11]:
- Infants up to 1 year 400 E (10 μg)/d
- Infants up to 3 years 600 E (15 μg)/d
- Persons between 3 and 60 years, pregnant and lactating women 600 E (15 μg)/d
- Persons over 60 years 800 E (20 μg)/d
“Supply” refers to all sources of vitamin D (diet and supplements), assuming limited sun exposure (e.g., individuals in institutions such as homes, in European winter climates, using sunscreens, etc.).
Calcium
How should we now proceed in family practice? First, a word about calcium. With a good supply of vitamin D, oral intake of calcium from 700-800 mg/d no longer leads to a further decrease in PTH [1]. So: with a good supply of vitamin D, a daily intake of 800 mg of calcium is considered sufficient. The Institute of Medicine (IOM) writes in its media release of 30.11.2010 that people over 50 years of age need 800-1000 mg calcium per day and therefore recommends to aim for an intake of 1000-1200 mg per day (the total intake, i.e. diet plus supplements, should not exceed 2000 mg) [12].
In practice: if the calculated calcium intake is 800-1000 mg/d or more, the recommended target is achieved. If it is less, you should optimize dairy products and mineral water. If this is not possible, a calcium supplement is needed. This should not usually exceed 500 mg; the preparation should be taken after a main meal and not on an empty stomach. Calcium carbonate should not be used in atrophic gastritis or long-term PPI therapy because it is not soluble in the alkaline environment. Individuals with PPI [13] or long-term steroid therapy [14], status post gastric bypass surgery, active inflammatory bowel disease and maldigestion/malabsorption or oxalate kidney stones need a higher calcium intake of 1500 to a maximum of 2000 mg.
In most studies on osteoporosis prophylaxis, calcium was investigated in combination with vitamin D, without considering individual intakes and thus the influence of dietary calcium. The efficacy of calcium supplements in individuals who take sufficient dietary calcium has not been established. Calcium supplementation alone without concomitant vitamin D administration is no longer recommended because an increased rate of hip fractures has been observed in observational studies and in a meta-analysis [15]. Excessive calcium intake could also increase cardiovascular risk [16].
Vitamin D
Basically, all inhabitants of Switzerland have at least seasonal vitamin D deficiency [6,7]. For this reason, vitamin D measurements are not needed to justify vitamin D supplementation. How should vitamin D be supplied to the organism? The easiest way to administer vitamin D would be through natural heliotherapy: two to three times a week, expose a quarter of the body surface (face, hands, and parts of arms and legs) to the sun for 5-25 minutes between 11 a.m. and 3 p.m., depending on skin type and season [1]. However, many individuals will avoid this because of the risk of cancer. In addition, the flat incidence of the midday sun in winter (except in the high mountains) prevents sufficient vitamin D production.
In general, vitamin D should be administered orally. We give 800 E cholecalciferol per day for seniors. Higher doses should be justified by deep vitamin D level measurements. In long-term therapy, doses up to 4000 E/d are considered safe for health. The aqueous-alcoholic and oily vitamin D3 solutions are suitable. Attention: The composition of the preparations is not uniform, which must be taken into account when dosing! One drop of the aqueous-alcoholic solution contains 100 units, but one drop of the oily solution Wild or Burgerstein contains 500 units. A pipettable oily solution from the Streuli company was newly introduced. Vitamin D is inexpensive (approx. 0.08 Fr./d). In Switzerland, only the alcoholic-aqueous ViDe3 drops 4500 E/ml from the company Wild are currently subject to compulsory health insurance coverage, the oily Streuli solution from November 2014.
With an elimination half-life of just over one month, vitamin D can be given daily, weekly, or monthly with a meal. It is worth noting that the daily administration costs of up to Fr. 5.- by Spitex or homes can massively exceed the price of the preparation itself. Therefore, for people who are unable to straighten the medication themselves, it is recommended to administer it monthly in orange juice or water (beware of alcohol content) or on a toast when administering the oily solution.
For patients who are unable to cooperate and have increased needs, an ampoule of 300,000 units i.m. every four months may be a cost-effective (approx. 5.- Fr./year) alternative (corresponds to approx. 2500 E/d). The oily i.m. injection results in a natural depot effect. Occasionally, however, painful granulomas are said to result. These can also lead to delayed absorption and effect. According to recent studies, administering high doses of, for example, 500,000 E only once a year can lead to increased falls and hip fractures in elderly patients and should no longer be done [17].
Caveats – what to watch out for?
Administration of preparations containing active vitamin D such as Rocaltrol® or AT 10® should be left to the nephrologist or endocrinologist specialist. In the case of narrow therapeutic application with the risk of hypercalcemia, the use of these substances is indicated and justifiable only in defined endocrinological and nephrological diseases.
In case of insufficient dietary calcium intake, the supplement should not exceed 500 mg. The patient must be instructed about taking it immediately after a main meal. Taken fasting, elevated calcium levels may result, which could promote calcium deposits (tissue calcinosis, arteriosclerosis). In a diet rich in oxalic acid (spinach, rhubarb, chard), calcium absorption is impaired because calcium oxalate complexes are formed and excreted in the feces, which is of course desirable for kidney stone prophylaxis, but limits the calcium available to the organism.
Individuals who remain symptomatic despite vitamin D and calcium supplementation could have magnesium deficiency syndrome, especially in renal patients and patients taking diuretics. This, in addition to neuromuscular complaints, leads to impaired PTH secretion, which in turn is associated with decreased stimulation of 1α-hydroxylase and thus may lead to impaired synthesis of active vitamin 1,25(OH)2D3 [18]. In addition to calcium, magnesium and vitamin D, the supply of calories and proteins as well as other essential micronutrients must be ensured in sufficient quantities. It is still desirable to have as active a leisure time as possible with weight-bearing exercises, ideally performed outdoors. This has been shown to be associated with positive effects on bone metabolism and vitamin D synthesis.
The future may lie in the direct administration of calcidiol, or 25(OH)D3. However, a commercial preparation is not yet commercially available. Calcidiol is the most hydrophilic substance among the vitamin D derivatives. Its absorption does not depend on fatty food. It also does not need to be hepatically hydroxylated at position 25 first; with this preparation, the vitamin D balance could be predictably normalized within hours.
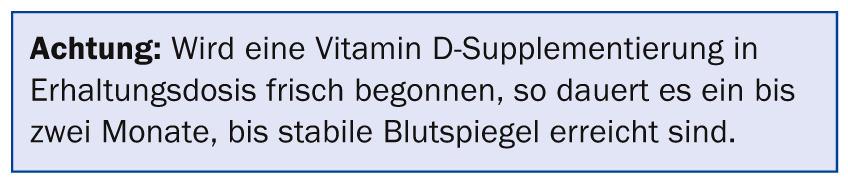
Trust is good – control is better
Normally – if indicated – supplementation should be used and not measured. Vitamin D levels need to be monitored only if symptoms persist despite proper supplementation or if there is doubt about the patient’s cooperation [1]. Routine determination should be performed if parathyroid surgery [19] or parenteral osteoporosis therapy are planned [20] to avoid the risk of symptomatic hypocalcemia. All other mirror measurements should be arranged specifically, e.g. in case of musculoskeletal problems, unclear symptoms, or for therapy control /motivation improvement.
For normal bone formation, 25(OH)D3 levels above 50 nmol/l are sufficient, but levels above 75 nmol/l are needed to optimize muscle strength and prevent falls in the elderly. Recommended supplement dosages are designed to achieve 25(OH)D3 levels above 50 nmol/l in 97.5% of individuals [21]. As mentioned earlier, seniors need higher levels if fall risk and muscle weakness are also to be effectively addressed. So, if we find a senior taking 800 E ViDe3 drops daily with good compliance to have a level of 50 nmol/l, the dose should be increased to 1500 E, for example, to minimize the risk of falls.
Special situations
For the treatment of high-turnover osteoporosis (rapid loss of bone density due to accelerated resorption), parenteral therapy with an i.v. bisphosphonate or denosumab (Prolia®) s.c. is often started. Both osteoporosis drugs have an antiresorptive effect and slow down accelerated bone loss. It should be noted, however, that in this situation there is usually also a pronounced vitamin D deficiency and thus a mineralization disorder in the sense of osteomalacia must be assumed. This is a contraindication for both therapeutic modalities [19]. First, the vitamin D metabolism must be normalized. If this is done with the maintenance dose of 800 E per day, it will take about two months to reach target levels. After that, it is necessary to wait another month until the osteoid is mineralized. Only then may antiresorptive osteoporosis therapy be started. This process can be accelerated by starting vitamin D therapy with a bolus. The loading dose (in units of cholecalciferol) can be calculated using the following formula: 40× (75 – current value 25(OH)D3 in nmol/l) × KG of the patient [22]. The bolus should usually be administered perorally after a fat-containing meal, followed by the maintenance dose. Then the specific osteoporosis therapy, which is not usually an emergency treatment, can be initiated after only one month. Patients should be advised that the risk of falls may increase for a short time immediately after administration of a vitamin D bolus (improved mobility due to increased muscle strength?) and thus fall prevention may be of particular importance [17].
A special situation arises in primary hyperparathyroidism. Most patients have severe vitamin D deficiency despite hypercalcemia. If this is not corrected preoperatively, life-threatening hypocalcemic crises may occur postoperatively. Interestingly, serum calcium barely increases with low-dose vitamin D supplements, whereas PTH may partially decrease despite the autonomy of the node [19].
An unsubstantiated assumption is that vitamin D supplements are contraindicated in patients with psoriasis and large-surface topical therapy with vitamin D analogues. Indeed, hypercalcemia can occur with large-scale topical psoriasis therapy, and calcium levels must be closely monitored with these therapies. However, most patients with psoriasis will be deficient in vitamin D, at least in winter, just like all other elderly residents of our country. This may also be the reason for the frequent flare-ups of psoriasis in winter. In any case, psoriasis patients over 60 years of age should also receive 800 E vitamin D per day according to the recommendations of the FOPH. Topical therapy is unable to achieve normal vitamin D levels and effects [23].
A similar problem arises in sarcoidosis. These patients have a higher than average incidence of reduced bone density and fragility fractures. They also have clustered hypercalciuria and/or hypercalcemia. Low 25(OH)D3 and high 1,25(OH)2D3levelsmay be a reflection of disease activity. Sarcoidosis per se is not an indication for vitamin D administration. However, a general recommendation to avoid vitamin D supplements in patients with sarcoidosis is not really supported empirically. Thus, vitamin D supplements, if otherwise justified (FOPH guidelines), may also be administered in these patients. Caution should probably be exercised for high doses of vitamin D. The levels of calcium, 25(OH)D3 and 1,25(OH)2D3 have to be monitored closely; for 25(OH)D3 a value between 50 and 75 nmol/l has to be aimed at, at which the risk for hypercalcemia does not seem to be increased yet [24].
Patients with malabsorption or maldigestion as a result of chronic inflammatory bowel disease or status post gastric bypass surgery, as well as those taking orlistat (Xenical®), often have fatty stools. These bind dietary calcium so that the formation of insoluble calcium oxalate complexes in the intestine is impaired. This leads to increased absorption of dietary oxalate. Such patients, especially if there is a history of kidney stones, require a low-oxalate diet and regular use of calcium supplements. Like all fat-soluble vitamins, vitamin D is absorbed in the jejunum and ileum. This is still possible in patients with status post bariatric surgery, so intramuscular injection is usually not required. However, sometimes patients have an increased requirement of 3000-6000 E/d [1].
Open questions remain
How is vitamin D deficiency really to be defined in different ethnic groups? Individuals of African descent have lower 25(OH)D3 levels but higher bone mass than Caucasians [25]. At what threshold does a decreased vitamin D level per se become a disease? What is the role of vitamin D-binding protein and vitamin D receptor pleomorphism? Not everything is clear by a long shot. Studies take time and money. The research industry is not prepared to pay the latter price in view of the low price and the lack of patent protection.
Conflicts of Interest: The authors have no conflicts of interest related to the content of this article.
Markus Gnädinger, MD
Literature:
- Holick MH: Vitamin D defiency. NEJM 2007; 357: 266-281.
- MacLaughin J, Holick FM: J Clin Invest 1985; 76: 1536-1538.
- Sato Y, et al: J Bone Mineral Res 2005; 20: 1327-1333.
- Holick MF: Am J Clin Nutr 1995; 61(suppl): 638S-645S.
- www.vitamindwiki.com/VitaminDWiki.
- Sakem B, et al: BMC Medicine 2013; 11: 176.
- Merlo C, et al: Practice 2012; 101(22): 1417-1422.
- Heijboer A, et al: Clin Chem 2012; 58(3): 534-538.
- Petersen AL: Maturitas 2014; 78(1): 40-44.
- Bischoff-Ferrari HA, et al: NEJM 2012; 367: 40-49.
- Vitamin D supply of the population: new recommendations of the FOPH of 20.6.2012: www.news.admin.ch
- IOM media release on calcium and vitamin D, Nov. 30, 2010.
- Tetsuhide I, Jensen RT: Curr Gastroenterol Rep 2010 December; 12(6): 448-457.
- Reid IR: Eur J Endocrinol 1997; 137: 209-217.
- Bischoff-Ferrari HA, et al: Am J Clin Nutr 2007; 86(6): 1780-1790.
- Bolland MJ, et al: BMJ 2011; 342: d2040.
- Sanders KM, et al: JAMA 2010; 303: 1815-1822. [Erratum, JAMA 2010; 303: 2357.]
- Kanazawa I, et al: Endocrine Journal 2007; 54(6): 935-940.
- Gnädinger M, et al: Switzerland Medicine Forum 2012; 12(34): 659-661.
- Gnädinger M, Mellinghoff HU: Switzerland Med Forum 2012; 12(37): 720-721.
- Holick MF, et al: J Clin Endocrinol Metab 2011; 96(7): 1911-1930.
- Van Groningen L, et al: Eur J Endocrinol 2010; 162: 805-811.
- Gnädinger M: Switzerland Med Forum 2013; 13(12): 262.
- Saidenberg-Kermanac’h N, et al: Arthritis Res Ther 2014; 16: R78.
- Harris SS: J Nutr 2006; 136(4): 1126-1129.
- Jehle S, et al: Swiss Med Wkly 2014; 144: w13942.
- Gnädinger M, Mellinghoff HU, Kaelin-Lang A: Swiss Med Wkly 2011; 141: w13154.
- FOPH: EEK vitamin D report: Ars Medici 2013; 3: 154-159.
CONCLUSION FOR PRACTICE
- Although doubts have been expressed recently, vitamin D supplementation and determination of 25-hydroxyvitamin D3 in high-risk groups represent a cost-effective, tolerable, and well-established practice.
- In particular, it can effectively prevent falls and fractures in elderly patients.
- The correlative associations found in observational studies with a whole range of diseases require corroboration by intervention studies.
HAUSARZT PRAXIS 2014; 9(12): 24-32