Herpes zoster is a burdensome and potentially dangerous disease, but now efficiently and readily preventable through vaccination. The recombinant adjuvanted zoster vaccine provides effective prophylaxis for patients. Preventive health care for patients is therefore a top priority for physicians. for patients high up on the list of priorities.
Often dismissed as a harmless childhood disease, varicella is frequently and regularly associated with secondary bacterial infections. Also known are severe, sometimes life-threatening hemorrhagic efflorescences and organ involvement in immunodeficiency. The risk of endogenous VZV reactivation (herpes zoster) already exists in childhood, typically whenever a child contracts varicella in the first months of life. However, varicella is not exclusively a childhood disease. Those who do not become infected in childhood will do so sooner or later in adulthood, with unpleasant consequences. This is because a disease in adulthood is usually more severe and is often accompanied by complications. Especially during pregnancy, contracting varicella should be avoided, as it can harm the fetus and, in the worst case, lead to stillbirth.
Pathogenesis
Initial infection with varicella zoster virus leads to immunity from re-infection by exogenous exposure on the one hand and protection from endogenous reactivation on the other. Presumably, some boosting of the immune system occurs with renewed contact with the virus or with herpes zoster itself. However, this natural boosting is not very efficient, which is why more and more reactivations are observed with increasing age (Fig. 1) [1].

The reason for the occurrence of herpes zoster is the persistence of the varicella zoster virus. It is believed that after the lesions of the original rash have healed, in which the viruses spread hematogenously and are then maximally present in hundreds of efflorescences on the skin, they migrate back along the sensory nerves into the spinal cord, where they dwell in the posterior horn in a latency stage and remain for life. If immunity then decreases, for example due to medication, immunosuppression or natural aging of the immune system, the virus returns to the skin along these nerves and leads to the classic hemiparesis of herpes zoster.
Clinical presentation of herpes zoster
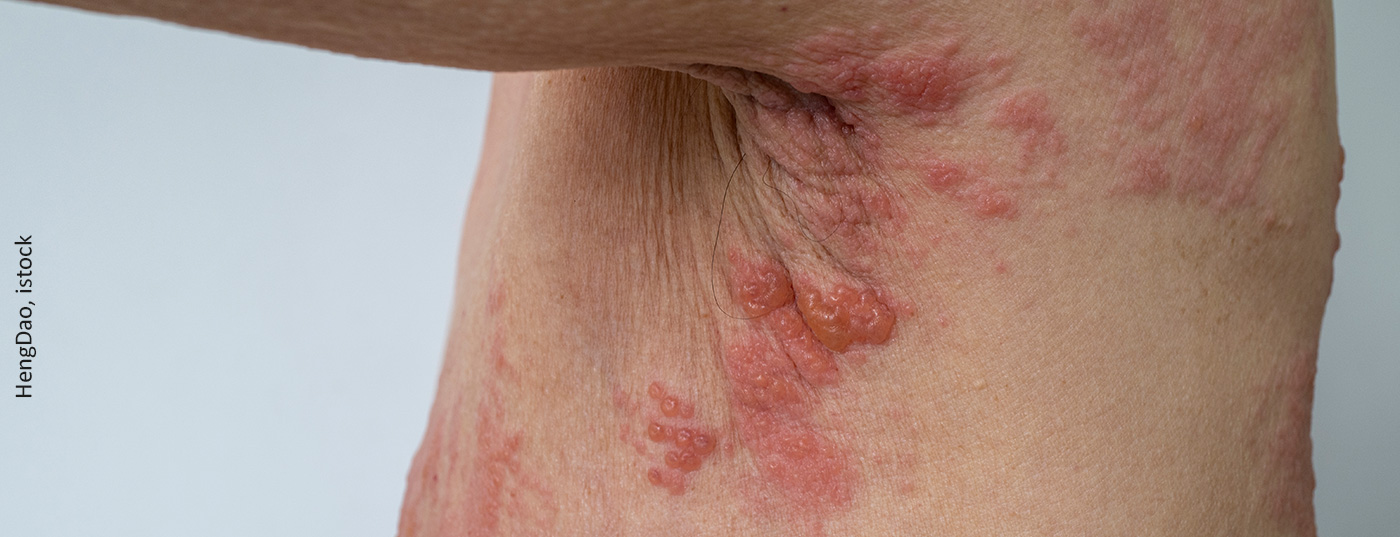
The clinical picture of herpes zoster manifests itself in painful efflorescences, grouped in the particular dermatome that undergoes reactivation. Herpes zoster is also feared for its post-herpetic neuralgia. The probability of living with pronounced pain for weeks or months after overcoming the disease correlates in turn with age. For disease at age 30, this affects 7% of patients; at age 50, it affects as many as 12%; and at age 70, it affects 18%. Also feared are ophthalmologic complications in the face, especially in the upper trigeminal section where the eye is involved. These can lead to corneal scarring and damage vision [2].
Epidemiology
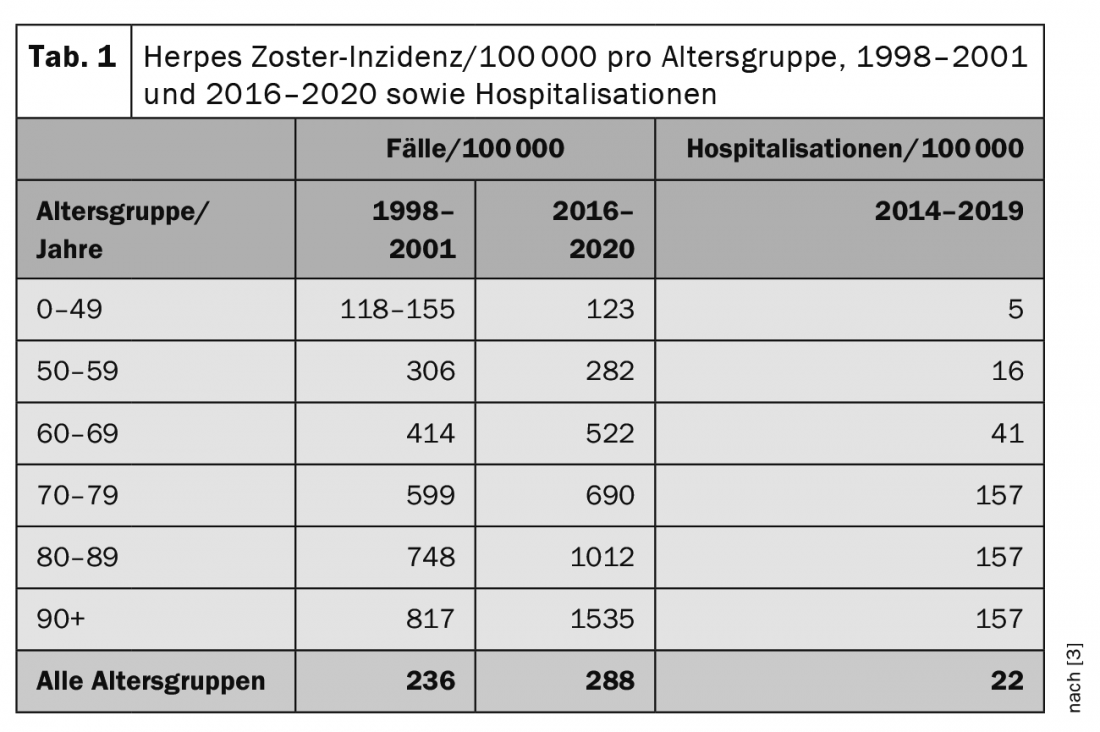
In Switzerland, the Swiss Sentinella surveillance system estimated the annual incidence of GP visits due to herpes zoster in the four years from 1998 to 2001. The results showed a stable average of about 17 000 cases per year. In the age group of 60 to 69 years, approximately 2700 cases per year were reported on an extrapolated basis; in the age group of 70 to 79 years, approximately 3000 cases per year; and in the age group of 80 years and older, approximately 2200 cases per year. Since 2016, herpes zoster has again been reported in the Sentinella surveillance system. (Tab. 1) [3] shows the extrapolated annual incidences for the two survey periods (mean values) per age group. Lifetime risk of contracting herpes zoster is cumulative. It is estimated that about 10-20% of all people develop the disease at least once in their lifetime. In addition, a significant increase in the number of herpes zoster cases can be seen in the last 10-20 years, especially in older people. The reason for this is probably that a number of treatment options of a drug nature (“biologics”) have been introduced in this age group in recent years. Although these enable more efficient treatment of, for example, systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA), they can lead to partial immunodeficiency as a side effect and thus increase the risk of herpes zoster. The risk of having to be hospitalized due to a severe course of herpes zoster therefore also correlates with age. A total of approximately 30,000 new cases of herpes zoster are recorded in Switzerland each year [3,4].
Vaccination prevention – “The queen of medicine
As early as 2018, the Federal Office of Public Health (FOPH) and the Swiss Federal Commission for Immunization Questions (EKIF) recommended vaccination against herpes zoster for the first time with a then novel live attenuated vaccine (Zostavax®) for the following two groups of persons: Immunocompetent persons aged 65 to 79 years and immunocompromised persons aged 50 to 79 years, provided they are not yet immunocompromised or are only slightly immunocompromised at the time of vaccination. Vaccination is given regardless of whether the person has already had varicella and/or herpes zoster. This live vaccine contains 14× more varicella zoster virus than Varivax® and is therefore so highly concentrated to boost the vaccinated person’s natural primary immunity. The recommended vaccination schedule is equivalent to one dose (0.65 ml s.c.) and is indicated for the prevention of herpes zoster and postherpetic neuralgia (PHN) caused by herpes zoster [5].
Live vaccine Zostavax®
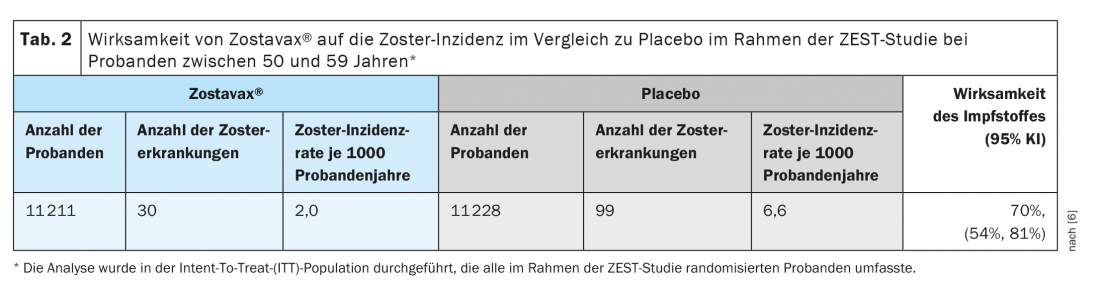
The protective clinical efficacy of the live vaccine has been demonstrated in two large randomized placebo-controlled clinical trials in which subjects received the vaccine subcutaneously. The ZEST trial was a placebo-controlled, double-blind clinical trial in which 22,439 subjects aged 50 to 59 years were randomized to receive a single dose of the vaccine or placebo and were followed for a median of 1.3 years (from 0 to 2 years) for zoster development. Final determination of zoster cases was made by polymerase chain reaction (PCR) (86%) or by clinical evaluation panel (14%). Zostavax® produced a significant decrease in zoster disease with an efficacy of 70% compared to placebo (Table 2) [6].
The SP trial was also a placebo-controlled, double-blind clinical trial in which 38,546 subjects aged 60 years and older were randomized to receive a single dose of the vaccine or placebo and were followed for a median of 3.1 years (from 31 days to 4.9 years) for zoster development. In the SP study, although zoster decreased in almost all dermatomes, so did vaccine efficacy. This was in the range of 50-60% for those aged 60-69 years or even well below 50% from the age of 70 years (Table 3) [6].

Shingrix® inactivated vaccine
Recently, a further development of the herpes zoster vaccine, which has been administered internationally for some time, was introduced in Switzerland. This is the inactivated vaccine Shingrix®, which contains 50 µg of varicella-zoster virus glycoprotein E (gE antigen) + AS01B adjuvant. The AS01B adjuvant consists of the plant extract Quillaja saponaria Molina, fraction 21 (QS-21) (50 µg) and 3-O-desacyl-4′-monophosphoryl lipid A (MPL) from Salmonella minnesota (50 µg). The recommended vaccination schedule is equivalent to two doses (0.5 ml i.m.) two months apart and is indicated for the prevention of herpes zoster 18 years of age and older.
As with all viral infections, human immunity is directed against components on the surface of the varicella-zoster virus that are presented by immunodominant cells after infection. Glycoprotein E is a spinous process-like protein on the surface of varicella-zoster virus and is the main target for both humoral and cellular immune responses against the virus [7].
In a dose-finding study, subjects received either 25, 50, or 100 µg adjuvanted of a herpes zoster vaccine candidate containing varicella zoster virus glycoprotein E (gE) with or without the adjuvant system AS01B. The frequency of gE-specific CD4+ T cells was >3-fold higher after two doses of all gE/AS01B formulations than after one dose of 100 µg gE/AS01B or two doses of 100 µg gE/saline. The frequencies were comparable after two doses of 25, 50, or 100 µg gE/AS01B. Serum anti-GE antibody concentrations were comparable and higher than in the other groups after two doses of 50 or 100 µg gE/AS01B. The immune responses persisted for at least 36 months. The reactivity of all gE/AS01B formulations was similar but higher than that of gE/salt. The results showed that the three formulations of gE/AS01B were immunogenic and well tolerated by adults aged ≥60 years. Moreover, two vaccinations with gE/AS01B induced higher immune responses than one, and the gE dose affected humoral but not cellular immune responses [8].
Efficacy and tolerability
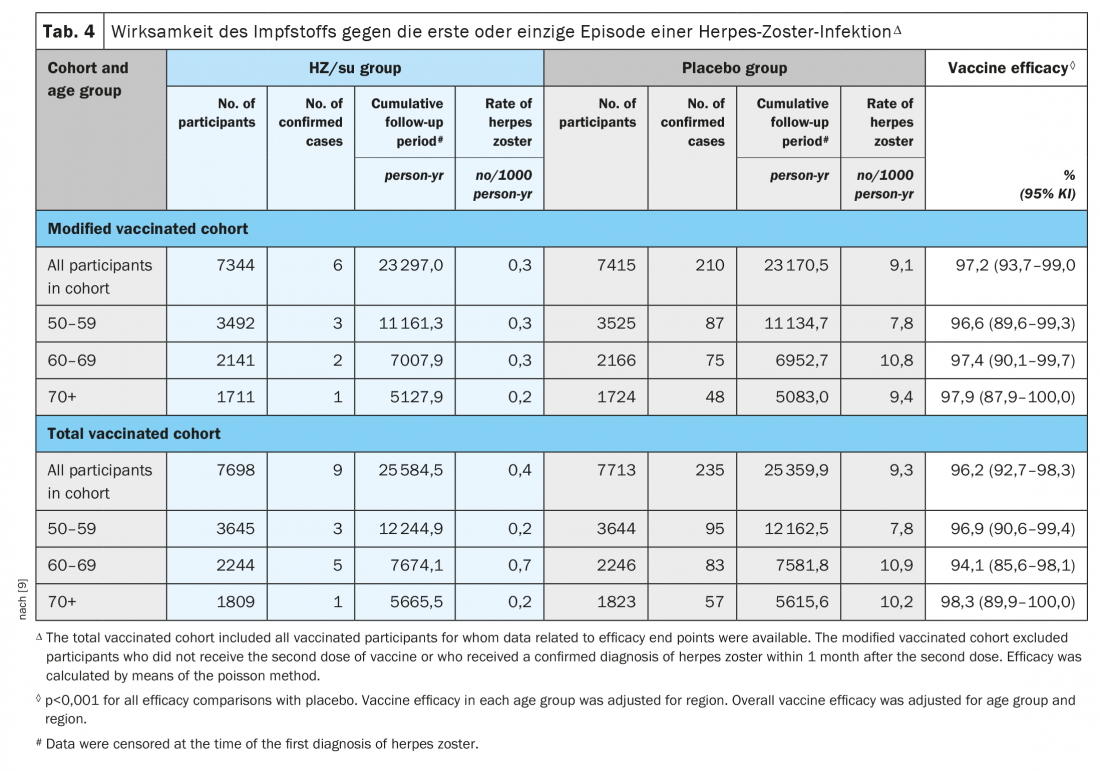
The ZOE-50 study of a total of 15,411 adults aged 50 years and older showed that herpes zoster subunit vaccine (HZ/su) containing recombinant varicella zoster virus glycoprotein E and adjuvant AS01B was associated with a 97.2% (95% confidence interval, 93.7 to 99.0; p<0.001) lower risk of herpes zoster than placebo. Vaccine efficacy ranged from 96.6% to 97.9% for all age groups (Table 4) [9].
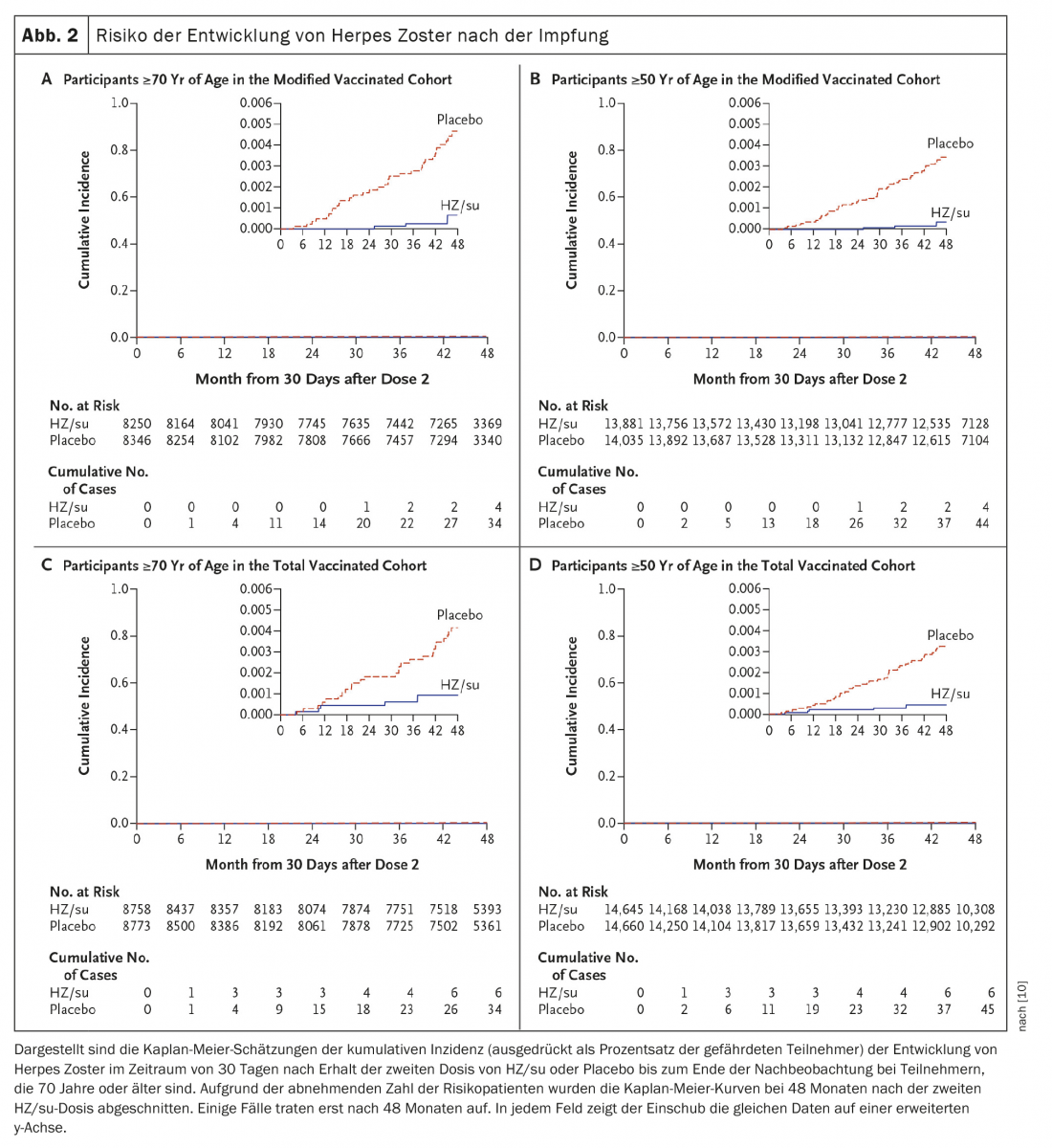
A second study was conducted concurrently at the same sites and examined the safety and efficacy of HZ/su in adults 70 years of age and older (ZOE-70). In ZOE-70, 13 900 evaluable participants (mean age 75.6 years) received either HZ/su (6950 participants) or placebo (6950 participants). During a median follow-up of 3.7 years, herpes zoster occurred in 23 HZ/su recipients and in 223 placebo recipients (0.9 vs. 9.2 per 1000 person-years). Efficacy of the herpes zoster vaccine was 89.8% (95% confidence interval, 84.2 to 93.7; p<0.001) and was similar in participants aged 70 to 79 years (90.0%) and participants aged 80 years or older (89.1%). In pooled analyses of data from participants aged 70 years or older in ZOE-50 and ZOE-70 (16 596 participants), vaccine efficacy against herpes zoster was 91.3% (95% CI, 86.8 to 94.5; p<0.001) and vaccine efficacy against postherpetic neuralgia 88.8% (95% CI, 68.7 to 97.1; p<0.001) (Fig. 2) [10].
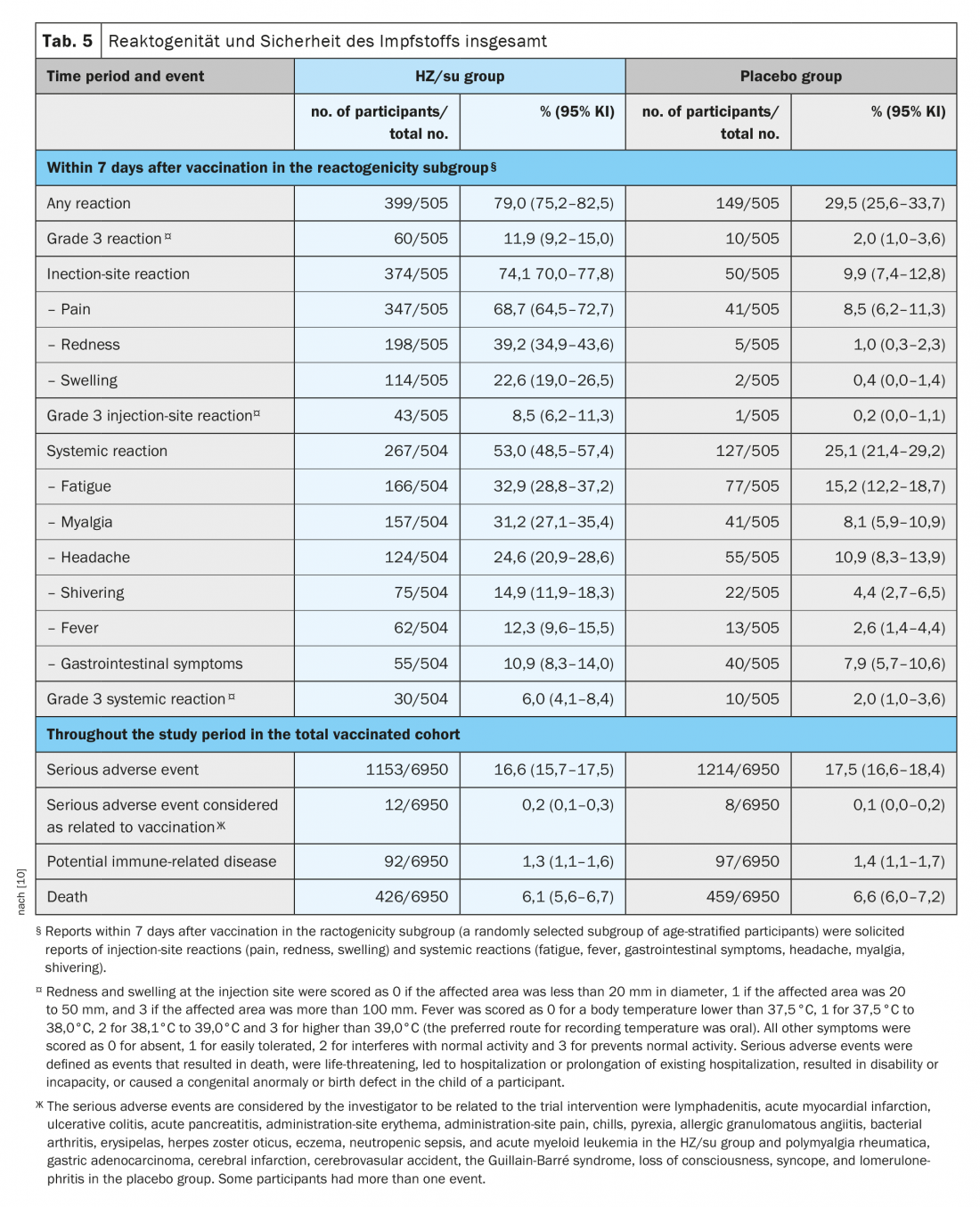
In the ZOE-70 study, a total of 1025 participants (7.4%) were randomly assigned to the reactogenicity subgroup (512 HZ/su recipients and 513 placebo recipients). In this subgroup, requested reports of reactions occurred within seven days of each vaccination in 79.0% of HZ/su recipients and in 29.5% of placebo recipients (Table 5) [10]. Whereas injection site reactions occurred in 74.1% of HZ/su recipients and in 9.9% of placebo recipients; most reactions were of mild to moderate intensity. Grade 3 injection site reactions were reported in 8.5% of HZ/su recipients and in 0.2% of placebo recipients. Systemic reactions occurred in 53.0% of HZ/su recipients and in 25.1% of placebo recipients (grade 3 reactions were reported in 6.0% and 2.0%, respectively). In the HZ/su group, the most common injection site reaction was pain (in 68.7% of HZ/su recipients) and the most common systemic reaction was fatigue (in 32.9%). Reactions were transient, with a median duration of two to three days for injection site reactions, one to two days for systemic reactions, and one to two days for grade 3 reactions. The overall frequency and severity of triggered reactions did not increase significantly after the second dose [10].
New recommendation for vaccination against herpes zoster
Based on these results, which are clearly positive compared to the live vaccine, the FOPH and EKIF recommend vaccination against herpes zoster with the adjuvanted subunit vaccine Shingrix® since 2021. This applies to healthy individuals 65 years of age and older, and to patients with immunodeficiency 50 years of age and older or with severe immunodeficiency 18 years of age and older. The previous November 2017 recommendations for the live vaccine Zostavax® now apply only to persons aged 65 to 79 years without immunodeficiency, who prefer Zostavax® to Shingrix® [3].
Shingrix® is recommended as a supplemental vaccine for all immunocompetent individuals ≥65 years of age, regardless of their individual history of varicella and herpes zoster. Physicians are required to educate their patients about this vaccination option. In addition, Shingrix® is recommended for all patients aged ≥50 years with current or future (especially cellular) immunodeficiency associated with an increased risk of herpes zoster. This applies, for example, to HIV-positive individuals or patients before, during or after active oncological therapy. In these patients, the first dose should ideally be administered ≥2 weeks before the start of chemotherapy. The second dose with a minimum interval of one to two months after the first dose or as soon as possible later during or after chemotherapy. Furthermore, patients with end-stage renal disease or on dialysis, on biologics, azathioprine, low-dose methotrexate, or low-dose corticosteroid maintenance therapy, as well as patients with other underlying diseases that impair immunity (especially cellular immunity) are affected. This includes, for example, patients with rheumatoid arthritis, severe asthma/COPD, inadequately controlled type 1 diabetes mellitus and other autoimmune diseases. In these individuals, two doses of Shingrix® are recommended with a minimum interval of two months.
In addition, Shingrix® is recommended for patients aged ≥18 years who are currently suffering from severe immunodeficiency or who are currently receiving or will receive immunosuppressive treatment in the foreseeable future. In this case, the first dose should ideally be administered ≥4 weeks before an assumed, expected, or planned onset of severe immunosuppression. The second dose with a minimum interval of one to two months after the first dose or as soon as possible at a later time during or after therapy that is favorable from a medical point of view. These include, for example, patients with hematologic malignancies, recipients of hematopoietic stem cells and organ transplants, individuals being treated with JAK inhibitors or intensive immunosuppression (e.g., combinations of immunosuppressants, high-dose corticosteroids) for an immune-mediated disease such as rheumatoid arthritis or inflammatory bowel disease, and HIV-positive individuals with <200 CD4 + T cells/l or <5% lymphocyte percentage.
In pregnant or lactating women, a careful individual risk-benefit assessment should be made. VZV serology (antibody testing) is not recommended prior to vaccination with Shingrix®. Neither Shingrix® nor Zostavax® should be used to prevent initial infection with VZV (varicella = chickenpox).
Recommendations for the live vaccine Zostavax®.
The currently available live vaccine Zostavax® is contraindicated in individuals with immunodeficiency and should be discontinued in individuals receiving immunosuppressive therapies in the near future. Zostavax® remains an option for immunocompetent individuals aged 65 to 79 years who prefer Zostavax® over Shingrix®. Zostavax® is not reimbursed by the OKP.
Take-Home Messages
- Herpes zoster is a burdensome and potentially dangerous disease, but now efficiently and readily preventable through vaccination.
- The recombinant adjuvanted zoster vaccine provides effective prophylaxis for patients.
- One of the tasks of physicians is therefore to place preventive health care for patients high on the list of priorities.
Literature:
- Arvin A: Immunity, and the Varicella-Zoster Virus. N Engl J Med 2005; doi: 10.1056/NEJMp058091.
- Federal Commission for Immunization Questions (EKIF): Evaluation of vaccines and vaccination against herpes zoster (ZOSTAVAX® and SHINGRIX®), 2021.
- Federal Office of Public Health FOPH: FOPH Bulletin 47/2021, issue of November 27, 2021.
- Bundesamt für Gesundheit = Office fédéral de la santé publique. Vaccination against herpes zoster: not included in the Swiss vaccination schedule. Bulletin BAG – OFSP 2010(6): 97.
- Federal Office of Public Health, Federal Commission for Immunization Issues (EKIF): Schweizerischer Impfplan 2018. Guidelines and Recommendations. Bern: Federal Office of Public Health, 2018.
- Specialty Information Zostavax®, available at: www.ema.europa.eu/en/documents/product-information/zostavax-epar-product-information_de.pdf.
- Zerboni L, et al: Molecular mechanisms of varicella zoster virus pathogenesis. Nat Rev Microbiol 2014; doi: 10.1038/nrmicro3215.
- Chlibek R, et al: Safety and immunogenicity of three different formulations of an adjuvanted varicella-zoster virus subunit candidate vaccine in older adults: A phase II, randomized, controlled study. Vaccine 2014; doi: 10.1016/j.vaccine.2014.01.019.
- Lal H, et al: Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N Engl J Med 2015; doi: 10.1056/NEJMoa1501184.
- Cunningham AL, et al: Efficacy of the Herpes Zoster Subunit Vaccine in Adults 70 Years of Age or Older. N Engl J Med 2016; doi: 10.1056/NEJMoa1603800.
HAUSARZT PRAXIS 2022; 17(10): 6-12