Helicobacter pylori infection induces chronic active gastritis. If the diagnostic findings are positive, eradication is recommended. The risk of resistance formation should also be taken into account when selecting a therapy. The range of approved second-line options has recently been expanded.
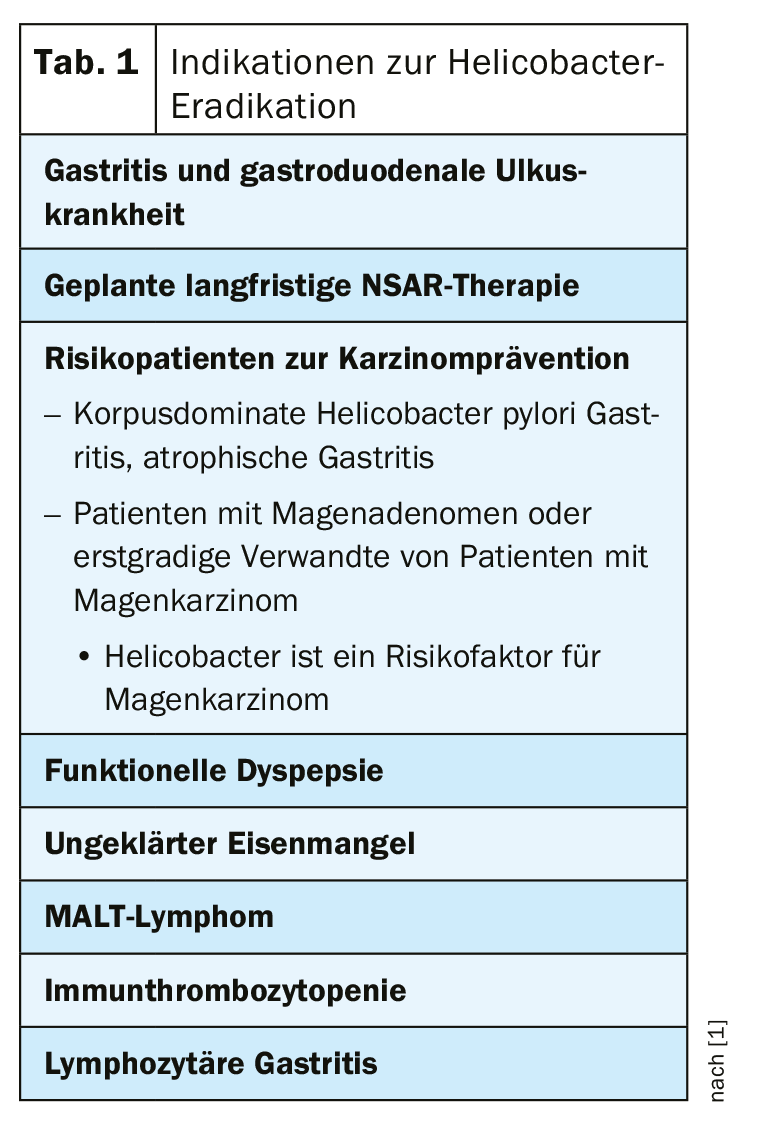
It is estimated that approximately 50% of the world’s adult population over the age of 40 is infected with Helicobacter pylori (H. pylori) [1]. H. pylori infection induces chronic active gastritis and various sequelae may occur (e.g., dyspeptic symptoms, gastroduodenal ulcer disease, distal gastric carcinoma) [2]. There is no gold standard for Helicobacter diagnostics in patients with upper abdominal complaints, explains Marcel Halama, MD, Gastroenterology Practice, Zurich [3]. If looking for Helicobacter, it should be noted that if treatment with proton pump inhibitors (PPI) is ongoing, they should be discontinued at least two weeks prior to testing. In case of a test repetition after eradication for control purposes, this should be done only after an interval of at least four weeks. Which method is used for Helicobacter diagnostics is a matter of discretion. If endoscopy is performed, the speaker recommends a rapid test procedure or histologic analysis. If endoscopy is not performed, stool antigen or breath tests are suitable methods, which are comparable in terms of cost, sensitivity and specificity. According to the speaker, if Helicobacter is detected, eradication therapy should always be performed. A summary of the indications for Helicobacter eradication is recorded in the 2016 guidelines of the German Society for Gasteroenterology, Digestive and Metabolic Diseases (DGVS) [1] (Table 1).
Multiple first-line therapy regimens available
The problem of the risk of resistance development mainly concerns metronidazole and clarithromycin [4]. Amoxicillin, on the other hand, very rarely leads to the development of resistance. If you are unsure which antibiotic to use, you can also use combined therapy. The eradication rate is about 93%, a disadvantage is the somewhat complicated handling for the patient due to the large number of drugs which have to be taken over a period of ten days [5–6]. Sequential therapy is another first-line treatment option; eradication rates of 81-91% have been achieved [5–6].
The speaker recommends one of the following schemes [3] as a standard action:
Standard therapy 1:
- PPI 2× standard dose/day
- Clarithromycin 2× 500 mg/day
- Metronidazole 2× 500 mg/day
Standard therapy 2:
- PPI 2× standard dose/day
- Clarithromycin 2× 500 mg/day
- Amoxicillin 2× 1000 mg/day
Combined or sequential treatment can be performed as follows [3]:
Combined therapy:
all for 5 days:
- PPI 2× standard dose/day
- Amoxicillin 2× 1000 mg/day
- Metronidazole 2× 500 mg/day
- Clarythromycin 2× 500 mg/day
Sequential therapy:
- for 5 days the following combination:
- PPI 2× standard dose/day
- Amoxicillin 2× 1000 mg/day
directly afterwards for 5 days:
- PPI 2x standard dose/day
- Metronidazole 2× 500 mg/day
- Clarithromycin 2× 500 mg/day
Pylera® has proven itself
In Switzerland, Pylera® [7] has been approved as a second-line treatment option since Sept. 1, 2018. In Germany, this preparation has already been on the market for several years and good eradication rates (>90%) have been achieved. One advantage is that this regimen does not include amoxicillin, so it can also be used to treat penicillin allergic patients [8,9]. There is no agreement on whether and according to which criteria a resistance search – a relatively complex procedure – should be carried out. The DGVS guidelines suggest analyzing for resistance after the second unsuccessful eradication attempt [1,3]. In Switzerland, there is no recommendation in this regard. If Helicobacter is found in the stool, a new treatment with second-line or third-line regimen is performed instead of another endoscopy [3].
Source: FOMF Zurich
Literature:
- Digestive and Metabolic Diseases (DGVS): Helicobacter pylori and gastroduodenal ulcer disease, 02/2016 S2k guideline, www.dgvs.de
- Malfertheiner P, Link A, Selgrad M: Helicobacter pylori: perspectives and time trends. Nat Rev Gastroenterol Hepatol 2014; 11: 628-638.
- Halama M: Slide presentation Marcel Halama, MD, Gastroenterology Practice, Zurich. Ulcer and reflux disease. FOMF Internal Medicine – Update Refresher, 04.12.2019, Zurich.
- Savoldi A, et al: Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018; 155(5): 1372-1382.e17.
- Wu DC, et al: Sequential and concomitant therapy with four drugs is equally effective for eradication of H pylori infection. Clin Gastroenterol Hepatol 2010; 8(1): 36-41.e1.
- Malfertheiner P, et al: Management of Helicobacter pylori infection – the Maastricht IV/ Florence Consensus Report. Gut 2012; 61(5): 646-664.
- Swiss Drug Compendium: Pylera®, https://compendium.ch
- Malfertheiner P, et al: Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017; 66(1): 6-30.
- Malfertheimer P, et al: Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011; 377(9769): 905-913.
HAUSARZT PRAXIS 2020; 15(2): 18 (published 6.2.20, ahead of print).