Recently, there have been significant advances in the treatment of atopic dermatitis, including several new systemic therapeutic options overcoming regulatory hurdles. This has also been incorporated into the EuroGuiDerm guideline on the treatment of atopic eczema published last year. Thus, detailed guidance is included on the use of biologics and JAK inhibitors.
Evidence-based guidelines provide a comprehensive overview of the latest evidence and are an important basis for decision-making in the selection of adequate treatment. The European Dermatology Forum (EDF) is committed to improving healthcare for dermatology patients across Europe and launched the European Centre for Guidelines Development several years ago. The European guideline for atopic eczema published in 2022 was developed according to the EuroGuiDerm standards described on the EDF website. In general, systemic therapy is considered indicated when moderate to severe atopic eczema cannot be adequately controlled with topical medications and UV light therapy. Systemic therapy may also be useful to allow patients who require prolonged use of topical corticosteroids (TCS) to control atopic eczema to reduce the amount of TCS [1].
Innovative highly effective substances – what is important to consider?
In the case of the two biologics dupilumab and tralokinumab, not only efficacy but also tolerability is rated as advantageous. No regular laboratory controls or other monitoring measures are required [2]. However, in contrast to the Janus kinase (JAK) inhibitors, which are characterized by a very rapid onset of action, Dupilumab and Tralokinumab require several weeks to develop their full efficacy [1]. JAK inhibitors also represent highly effective and safe therapeutic options, provided that contraindications, side effects, and necessary laboratory tests are taken into account. While the two biologics are applied subcutaneously with dosing intervals of 2-4 weeks, the JAK inhibitors are available in oral dosage form, with daily dosing recommended.
Biologics: highly effective and targeted – no routine monitoring required
The approval of the first biologic – dupilumab – was an important milestone and set new standards for the treatment of atopic dermatitis. In Switzerland, dupilumab (Dupixent®) has been approved for the treatment of moderate to severe atopic dermatitis (AD) in adults since 2019, followed by indication expansion for adolescents, and since June 2022, dupilumab can also be used for children aged 6 years and older [3]. Dupilumab binds to the α-subunit of the IL-4 receptor, which is part of both the IL-4 and IL-13 receptor complexes. In adults, the recommended starting dose is 600 mg dupilumab (two 300 mg injections) and the maintenance dose is 300 mg every two weeks. For children and adolescents from 6 to 17 years, the dosage recommendation depends on body weight.
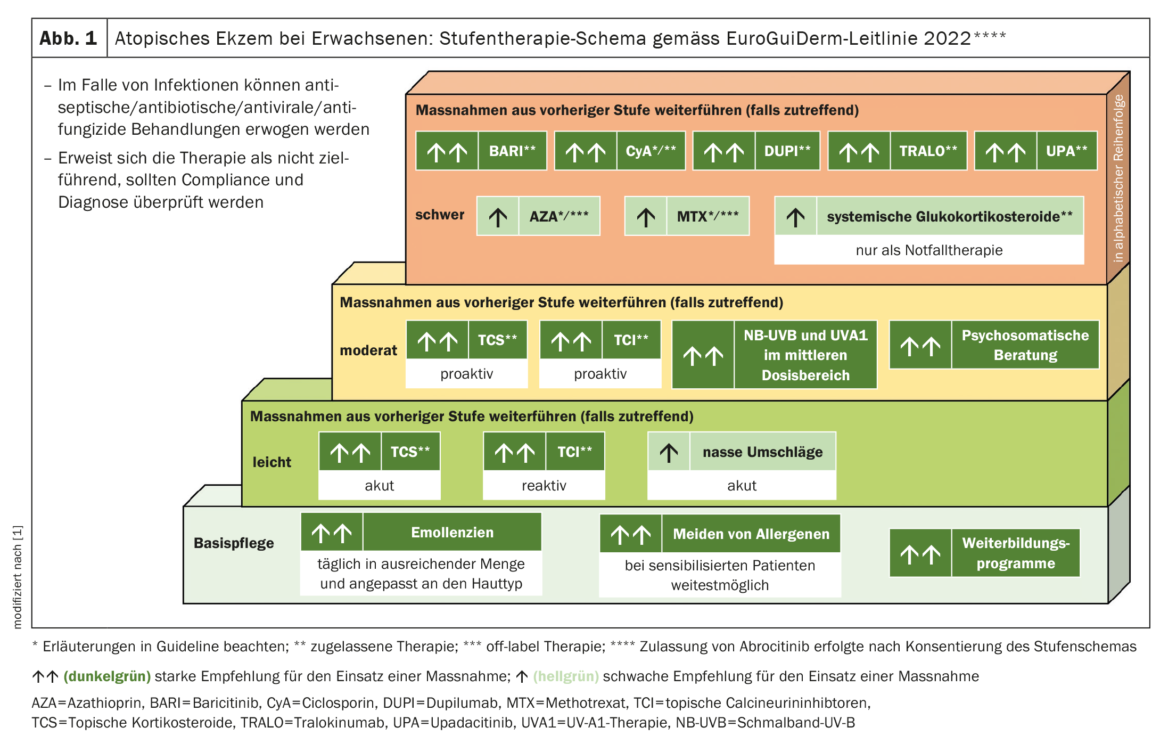
The EuroGuiDerm guideline indicates that treatment with dupilumab is generally well tolerated and no routine laboratory chemistry blood tests are required [1]. Conjunctivitis develops in about one-third of patients. However, this can be treated well. In most cases, the use of anti-inflammatory eye drops is sufficient and dupilumab treatment does not need to be interrupted [4–6].
Tralokinumab (Adtralza®) is also a human monoclonal antibody, but unlike dupilumab, it specifically neutralizes IL-13. In Switzerland, tralokinumab has been approved for adult patients with moderate to severe AD since June 2022 [3]. The initial dose for adults is 600 mg (4 injections of 150 mg each), followed by a maintenance dose of 300 mg (2 injections of 150 mg each), with a dosing interval of 2 weeks each. From week 16, the interval can be extended to 4 weeks. As with dupilumab, the EuroGuiDerm guideline mentions conjunctivitis as a possible side effect, although this occurs in a lower proportion of patients with tralokinumab [1,7]. As with dupilumab, routine laboratory blood tests are also not required [1].
JAK inhibitors: orally administrable and rapid onset of action.
The EuroGuiDerm guideline recommends the same investigations for baseline screening and treatment monitoring for all currently approved JAK inhibitors [1]. The guideline points out that the approval of abrocitinib followed consensus on the stepwise therapy regimen, which is why this JAK inhibitor is not listed there, but there are detailed explanations in the guideline for this therapy option as well. Common to all JAK inhibitors is the recommendation of baseline screening, including complete blood count, renal/liver/lipid profile, and creatinine phosphokinase levels, and hepatitis and tuberculosis screening (including chest x-ray). For monitoring, a complete blood count, renal/liver/lipid profile, and creatinine phosphokinase level are recommended after four weeks of treatment, or every three months during therapy.
Baricitinib (Olumiant®) is an oral selective JAK1 and JAK2 inhibitor approved in Switzerland for the treatment of adult patients with moderate-to-severe AD since February 2021 [3]. The standard dosage is 4 mg (1×/d p.o.). The 2-mg dose (1×/d p.o.) is considered in patients 75 years of age and older or in patients with chronic/recurrent infections or a creatinine clearance of 30-60 ml/min. The EuroGuiDerm guideline lists upper respiratory tract infections, headache, and an increase in LDL cholesterol as the most common side effects of baricitinib in clinical trials [1]. Acne occurs less frequently than with other JAK inhibitors. No malignancies were reported in baricitinib-treated patients during the placebo-controlled period, and no gastrointestinal perforations, positive cardiovascular events, or cases of tuberculosis were reported.
Upadacitinib (Rinvoq®) is a perorally bioavailable, selective and reversible JAK inhibitor that primarily inhibits JAK1 [3]. In Switzerland, upadacitinib has been approved for the treatment of moderate-to-severe AD in adults since November 2021. The recommended oral dose is 15 mg of upadacitinib once daily. According to the EuroGuiDerm guideline, upper respiratory tract infections and acne were the most common adverse events with upadacitinib in clinical trials [1]. The cumulative incidence rates of serious adverse events were 0% for 30 mg, 2.4% for 15 mg, 4.8% for 7.5 mg, and 2.4% for placebo.
Abrocitinib (Cibinqo®) is an oral selective JAK-1 inhibitor. In Switzerland, abrocitinib has been approved for the treatment of AD in adults since April 2022 [3]. In the EuroGuiDerm guideline, dose-related adverse events (200 mg, 100 mg, placebo) were nausea (14.6%, 6.1%, 2.0%), headache (7.8%, 5.9%, 3.5%), and acne (4.7%, 1.6%, 0%). In the 200 mg and 100 mg abrocitinib groups, the incidence rates for serious infections were 2.33/100 patient-years (PY) and 2.65/100 PY, respectively. For herpes zoster, incidence rates were 4.34/100 PJ and 2.04/100 PJ, respectively, and for herpes simplex, incidence rates were 11.83/100 PJ and 8.73/100 PJ, respectively [1,8].
| The development of the EuroGuiDerm guideline, published in the Journal of the European Academy of Dermatology and Veneorology , involved 26 experts from 12 European countries who were nominated by national partner societies or the two guideline coordinators. In addition, three patient representatives participated in the development of the guideline. Part 1 summarizes recommendations for systems therapy, and Part 2 focuses on non-systems treatment options. For Part 1 of the guideline, in addition to the recommendations of the expert panel, findings from the review by Drucker et al. considered. It should be noted that when applying the therapy recommendations, attention should be paid to local licensing conditions and circumstances. to [1,9] |
Other active substances mentioned in the guideline
In addition to conventional and new systemic therapy options, the EuroGuiDerm guideline also mentions the two drug candidates lebrikizumab and nemolizumab – both biologics that are currently being investigated for use in AD in phase III trials [1]. Lebrikizumab is a monoclonal antibody that specifically binds to freely circulating IL-13. Nemolizumab blocks the α-subunit of the IL-31 receptor. Other agents mentioned in the guideline include the anti-IgE monoclonal antibody omalizumab, which is approved for the treatment of asthma and chronic spontaneous urticaria but not for atopic eczema. The evidence base to date on omalizumab in atopic eczema is controversial. Another active ingredient mentioned is alitretinoin, which is approved in some European countries for the treatment of chronic hand eczema. Alitretinoin is an active ingredient from the group of retinoids.
Literature:
- Wollenberg A, et al: European guideline (EuroGuiDerm) on atopic eczema: part I – systemic therapy. J Eur Acad Dermatol Venereol 2022; 36(9): 1409-1431.
- Worm M, et al: Modern therapy of atopic dermatitis: biologics and small molecule drugs. J Dtsch Dermatol Ges 2020; 18(10): 1085-1093.
- Drug Information, www.swissmedicinfo.ch,(last accessed Mar. 08, 2022).
- Wollenberg A, et al: Laboratory safety of dupilumab in moderate-to-severe atopic dermatitis: results from three phase III trials (LIBERTY AD SOLO 1, LIBERTY AD SOLO 2, LIBERTY AD CHRONOS). Br J Dermatol 2020; 182: 1120-1135.
- Akinlade B, et al: Conjunctivitis in dupilumab clinical trials. Br J Dermatol 2019; 181: 459-473.
- Wollenberg A, et al: Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract 2018; 6: 1778-1780.e1
- Wollenberg A, et al: Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol 2021; 184: 437-449.
- Simpson EL, Silverberg JI, Nosbaum A, et al: Integrated safety analysis of abrocitinib for the treatment of moderate-to-severe atopic dermatitis from the phase II and phase III clinical trial program. Am J Clin Dermatol 2021; 22: 693-707.
- Drucker AM, et al: Systemic Immunomodulatory Treatments for Patients With Atopic Dermatitis: A Systematic Review and Network Meta-analysis. JAMA Dermatology, 01 Jun 2020; 156(6): 659-667.
DERMATOLOGIE PRAXIS 2023; 33(2): 32-33











