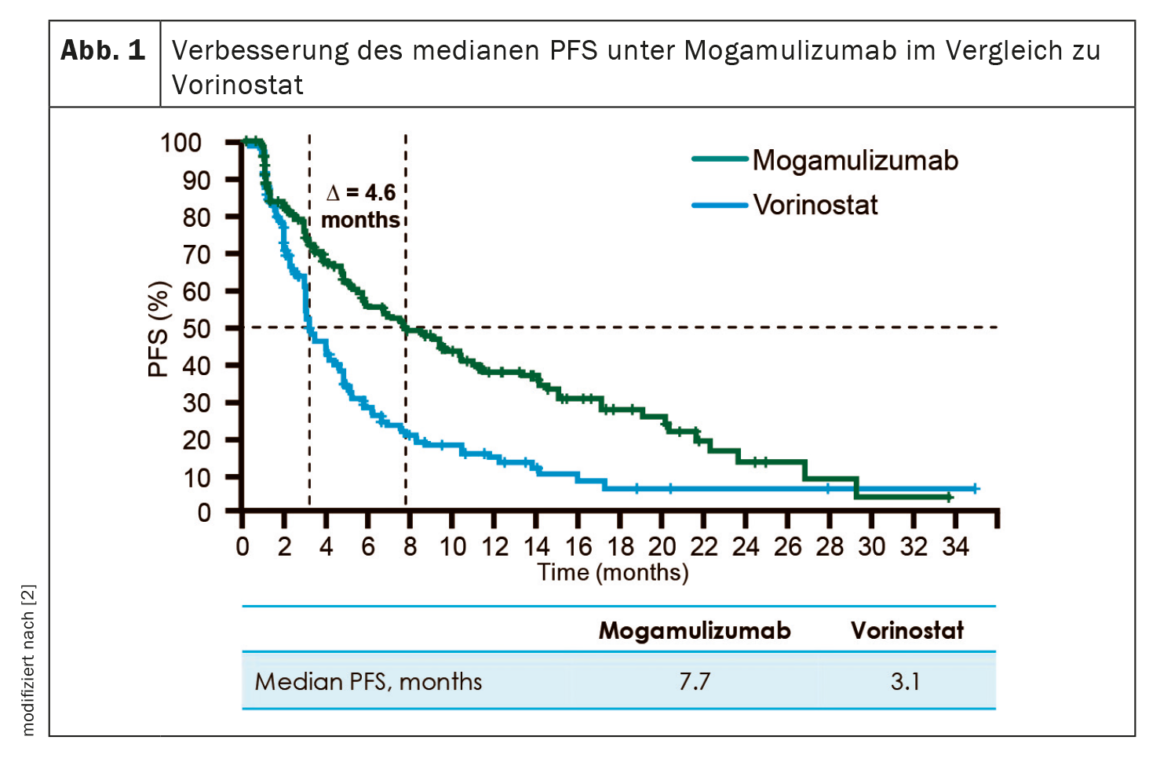
The approval of the anti-CCR4 antibody mogamulizumab provides patients with cutaneous T-cell lymphoma (CTCL) of the mycosis fungoides (MF) and Sézary syndrome (SS) subtypes with an effective non-chemotherapeutic intervention for the first time after prior systemic therapy. The innovative therapy option was able to demonstrate significantly improved progression-free survival and long-term response in the blood and skin compartments compared to comparator therapy.
Cutaneous T-cell lymphoma (CTCL) is characterized by migration of degenerate T cells into the skin [3]. The rare but serious disease can affect the skin, blood, lymph node and internal organ compartments [4,5]. The most common subtypes observed are MF and SS, as pointed out by Prof. Reinhard Dummer, MD, Zurich, Switzerland [3,5–7]. Red patches and plaques are typical and show similarities to other skin diseases and are accordingly often attributed to psoriasis or eczema [8]. Therefore, an average of two to seven years elapse until diagnosis – valuable time during which the disease can progress [8]. This is because even in early stages, up to 34% of MF patients experience disease progression [7]. In the advanced stage, only 52% of those affected survive longer than five years [9].
High demand for effective therapy
The earliest possible diagnosis of CTCL is crucial to initiate effective therapeutic management. In addition to the increasing risk of mortality, patients also suffer from a massive impairment of quality of life, according to the expert [10]. The goal of therapy should therefore be remission in the affected compartments, symptom relief, and minimization of disease progression [11].
Anti-CCR4 antibody approved
Mogamulizumab (Poteligeo®) has now been approved in Switzerland as the first antibody therapy for adult CTCL patients with MF or SS who had received at least one prior systemic therapy [1]. The approval is based on the results of the phase III MAVORIC trial [2]. Randomized 1:1, 372 sufferers received either mogamulizumab 1.0 mg/kg i.v. weekly for the first 28 days and on day 1 and day 15 in subsequent cycles, or vorinostat 400 mg daily (not approved in Switzerland). As explained by Prof. Emmanuella Guenova, MD, Lausanne, Switzerland, mogamulizumab more than doubled the median PFS compared with vorinostat (7.7 months vs. 3.1 months) (Fig. 1) [2]. In addition, antibody therapy resulted in a higher overall response – particularly in the skin (41.9% vs. 15.6%) and blood (66.9% vs. 18.4%) compartments [1]. The median duration of response was 20.6 and 25.5 months, respectively [2]. In 80% of the patients, an improvement of the quality of life could be achieved in the 11th therapy cycle [12]. Overall, mogamulizumab treatment was well tolerated and demonstrated a manageable safety profile [2,13]. The most commonly reported adverse effects were infusion-related reactions (30%) and drug rash (21.5%) [1].
Source: Kyowa Kyrin
Literature:
- Poteligeo® Technical Information, as of May 2022 at
www.swissmedicinfo.ch. - Kim YH, et al: Mogamulizumab versus vorinostat in previously treated cutaneous T-cell lymphoma (MAVORIC): an international, open-label, randomised, controlled phase 3 trial. Lancet Oncol 2018; 19(9): 1192-1204.
- Nicolay JP, et al: CCR4 in cutaneous T-cell lymphoma: Therapeutic targeting of a pathogenic driver. Eur J Immunol 2021; 51: 1660-1671.
- National Organization for Rare Disorders: Rare Disease Database: Cutaneous T-Cell Lymphomas. Available at: https://rarediseases.org/rare-diseases/cutaneous-t-cell-lymphomas (last accessed 04.11.2022).
- Krejsgaard T, et al. Malignant inflammation in cutaneous T-cell lymphoma – a hostile takeover. Semin Immunopathol 2017;39(3): 269-282.
- Dippel E, et al: S2k – Guideline – Cutaneous Lymphomas (ICD10 C82 – C86) As of 06/30/2021. Available at: www.awmf.org/uploads/tx_szleitlinien/032-027l_S2k_Kutane_Lymphome_2021-12.pdf (last accessed 11/04/2022).
- Agar N, et al: Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31): 4730-4739.
- CL Foundation: A Patient’s Guide. Available at: www.clfoundation.org/sites/default/files/2018-04/a_patients_guide.pdf (last access 04.11.2022)
- Scarisbrick JJ, et al: Cutaneous Lymphoma International Consortium Study of Outcome in Advanced Stages of Mycosis Fungoides and Sézary Syndrome: Effect of Specific Prognostic Markers on Survival and Development of a Prognostic Model. J Clin Oncol 2015; 33(32): 3766-3773.
- Demierre MF, et al. Significant impact of cutaneous T-cell lymphoma on patients’ quality of life: results of a 2005 National Cutaneous Lymphoma Foundation Survey. Cancer 2006; 107(10): 2504-2511.
- Shalabi D, et al: Immune evasion and current immunotherapy strategies in mycosis fungoides (MF) and Sézary syndrome (SS). Chinese Clin Oncol 2019; 8(1): 11.
- Porcu P, et al: Quality of Life Effect of the Anti-CCR4 Monoclonal Antibody Mogamulizumab Versus Vorinostat in Patients With Cutaneous T-cell Lymphoma. Clin Lymphoma Myeloma Leuk. 2021; 21(2): 97-105.
- Afifi S, et al. A drug safety evaluation of mogamulizumab for the treatment of cutaneous T-Cell lymphoma. Expert Opin Drug Saf. 2019; 18(9): 769-776.
InFo ONCOLOGY & HEMATOLOGY 2023; 11(2): 19