At the 5th Dermatological Training Days in the Kongresshaus Zurich, the so-called “What’s New” sessions took place on Friday afternoon, in which current research results, therapy and diagnostic approaches were discussed. Among others, the topics were atopic dermatitis, psoriasis and melanoma. Pediatric aspects of dermatology were also part of the lecture series.
PD Dr. med. Alexander Navarini, University Hospital Zurich, presented some exciting examples from current research:
Ivermectin in rosacea [1]: In this randomized investigator-blinded phase III trial, topical ivermectin 1% once daily was shown to be significantly more effective than metronidazole 0.75% twice daily for papulopustular rosacea, and after a shorter time. The primary endpoint was the reduction of inflammatory lesions.
Moisturizer in neonates [2]: 118 neonates at high risk for atopic dermatitis were randomized to two groups. Some put moisturizer on daily, others do not. In the first group, 32% fewer newborns actually developed atopic dermatitis than in the comparison arm. Treatment had no effect on the development of allergic sensitization to protein (measured by specific IgE). However, such occurred significantly more often in children with atopic dermatitis/eczema.
Dupilumab in atopic dermatitis [3]: Dupilumab inhibits IL-4 and IL-13 and has already been shown to be effective in asthma. It now appears that the so-called EASI (Eczema Area and Severity Index) can be reduced with dupilumab similarly well as the PASI in psoriasis studies: 85% of patients with moderate to severe atopic dermatitis achieved an EASI50 (vs. 35% under placebo, p<0.001).
combination of nivolumab and ipilimumab [4]: In untreated metastatic melanoma, nivolumab alone or in combination with ipilimumab is significantly more effective than ipilimumab alone. Median progression-free survival values were 6.9 months, 11.5 months, and 2.9 months in the above treatment sequence.
News on psoriasis
Prof. Dr. med. Lars French, University Hospital Zurich, spoke on the topic of “Psoriasis”. What are the new developments in this area?
Apremilast [5]: Apremilast is an orally administered small molecule that inhibits phosphodiesterase-4. In the phase III trial called ESTEEM 1, the compound has now been studied in moderate to severe plaque psoriasis. Significantly more patients in the apremilast group (33.1%) achieved a PASI75 at 16 weeks than on placebo (5.3%). The PASI75 response curve of the crossover group that switched from placebo to apremilast after 16 weeks resembled that of the initial apremilast group by week 32. In addition, quality of life (DLQI) improved significantly under the test substance, as did pruritus and nail/scalp psoriasis. Among the common side effects, diarrhea, nausea, and headache are most notable. “In total, more than 4000 patients have been observed in phase II and III studies of apremilast. In both psoriasis and psoriatic arthritis, efficacy can now be considered assured, with a convincing safety profile. Monitoring is not required. Swissmedic approval is currently underway. It is likely to be indicated in patients with moderate to severe plaque psoriasis who have not responded to, or are intolerant of, or are not allowed to receive at least one systemic therapy,” said Prof. French.
Secukinumab [6]: IL-17 is a key cytokine in psoriasis. Secukinumab is a human monoclonal antibody that binds to and blocks IL-17A. Two phase III trials called FIXTURE and ERASURE showed that subcutaneous secukinumab was significantly more effective than placebo as well as etanercept at both 300 mg and 150 mg doses. A PASI75 response was achieved in ERASURE by 81.6% (300 mg), 71.6% (150 mg), and 4.5% (placebo) at week 12 of treatment. In FIXTURE, these were 77.1% (300 mg), 67.0% (150 mg), 44.0% (etanercept), and 4.9% (placebo). Response occurred significantly faster in the secukinumab group than in the etanercept arm. Infections were found more frequently than with placebo, but the rate was comparable to that with entanercept. Secukinumab is approved in Switzerland as Cosentyx®. It is indicated for the treatment of adult patients with moderate-to-severe plaque psoriasis who have not responded to, are contraindicated to, or are intolerant of systemic therapy including ciclosporin, methotrexate, acitretin, or PUVA/UVB. If therapeutic success has not been achieved after twelve weeks, treatment should be discontinued. The dose is 300 mg at week 0, 1, 2 ,3 4 and then every four weeks.
Molecular genetic testing in melanoma
According to PD Dr. med. Katrin Kerl, University Hospital Zurich, the spectrum of melanocytic tumors is broad. To be mentioned are:
- Melanoma in actinic/non-actinic
- damaged skin
- Acral melanoma
- Mucosal melanoma
- Childhood melanoma
- Spitzoid melanomas
Spitzoid melanocytic proliferations include atypical Spitz nevus, atypical Spitz tumor (intermediate malignant potential, no distant metastases), and precisely spitzoid melanoma (completely malignant, distant metastases). Currently, there are no reliable histopathologic criteria or molecular markers to distinguish borderline malignant from definite malignant neoplasms.
For molecular genetic testing in melanoma diagnosis, fluorescence in situ hybridization (FISH) is added, which has a specificity of 96% and sensitivity of 87% in conventional melanoma. However, for atypical Spitz tumors, sensitivity is 40-70%. A positive FISH does not necessarily mean melanoma. An additional probe for chromosome 9p21 improved the detection of spitzoid melanomas. Because: homozygous 9p21 deletion correlates with an aggressive course in atypical Spitz tumors.
In the meantime, the so-called TERT promoter gene mutations have also been discovered. TERT controls the activity of telomerase. Their activation leads to replicative immortality of cells. TERT promoter mutations are very common in various neoplasms, including melanoma. Here, along with BRAF and NRAS, they are among the most common mutations and are associated with poor outcome [7]. Therefore, researchers now wanted to find out whether they also have prognostic significance in spitzoid neoplasms [8]. They studied atypical spitzoid tumors or spitzoid melanomas from a total of 56 patients. None of the individuals with favorable outcome had such a mutation, whereas four patients with hematogenous metastases and lethal outcome all showed a TERT promoter mutation. The authors conclude that the presence of such a gene variant indicates a high-risk group within patients with spitzoid tumors. Thus, it is a potential routine marker of poor prognosis not only in melanoma but also in spitzoid tumors.
Pediatric dermatology
Atopic dermatitis in childhood: “Can skin barrier function in early childhood be used to predict which children will later develop eczema?” was the introductory question posed by Martin Theiler, MD, University Children’s Hospital Zurich. The answer is yes: an Irish study [9] of 1903 newborns demonstrated that high transepidermal water loss was a significant predictor of atopic dermatitis at one year of age, both two days after birth and two months later. At both time points, this effect was independent of any parental atopy or filaggrin mutation status. Children with high water loss sometimes had up to a threefold increased risk of atopic dermatitis. Together with the finding mentioned in the previous presentation that emollients provide protection in neonates (30-50% risk reduction) [2,10], the results highlight the relevance of the epidermal skin barrier in the prevention of the condition.
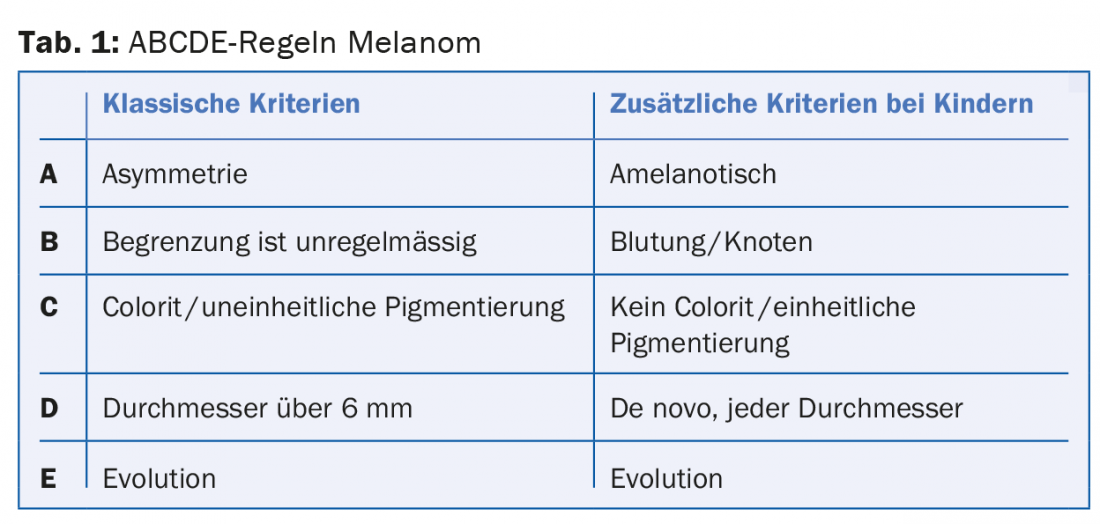
Childhood melanoma: “Regarding the clinical presentation of childhood melanoma, 60% of all melanomas in the first decade are missed according to the classic ABCDE rules,” cautioned Dr. Theiler (Tab.1). In principle, although melanoma is rare in this population, it is the most common malignant skin tumor in childhood and usually arises de novo, without identifiable risk factors. In early childhood, all ethnicities are affected at about the same rate, then with increasing age, children of Caucasian descent are affected much more frequently. The forecast is controversial.
Childhood tinea capitis: the British Association of Dermatologists issued new guidelines for the management of tinea capitis in 2014 [11]. For Trichophyton tonsurans/violaceum/soudanense, the use of terbinafine (Lamisil®, Myconormin®, Terbifil®, Terbinafin®, Tineafin®, and Terbinax®) is recommended. The tablet is mortarable. However, because controlled experience in children under 20 kg (usually <5 years of age) is still sparse, use should only be made in the absence of a therapeutic alternative if the benefits outweigh the presumed risks. The dose here is 62.5 mg/d. In children 20-40 kg it is 125 mg/d and in adolescents over 40 kg and in adults it is 250 mg/d. For Microsporum canis/audouinii, among others, itraconazole is used as a suspension that should be taken fasting (5 mg/kg bw per day as a single dose). Safety in the first year of life was demonstrated in a study [12]. Treatment is given until cultural pathogen detection is negative.
Camouflage: “Camouflage can also be a viable option for children,” says Dr. Theiler. This was shown in a study [13] of 38 children aged 5-18 years who had visible skin problems. Result: The Children’s Dermatology Life Quality Index (CDLQI) was significantly reduced with camouflage from an initial 5.1 to 2.1 (after six months).
Source: 5th Zurich Dermatology Training Days, June 24-26, 2015, Zurich.
Literature:
- Taieb A, et al: Superiority of ivermectin 1% cream over metronidazole 0-75% cream in treating inflammatory lesions of rosacea: a randomized, investigator-blinded trial. Br J Dermatol 2015 Apr; 172(4): 1103-1110.
- Horimukai K, et al: Application of moisturizer to neonates prevents development of atopic dermatitis. J Allergy Clin Immunol 2014 Oct; 134(4): 824-830.e6.
- Beck LA, et al: Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med 2014 Jul 10; 371(2): 130-139.
- Larkin J, et al: Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015 May 31 [Epub ahead of print].
- Papp K, et al: Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol 2015 Jul; 73(1): 37-49.
- Langley RG, et al: Secukinumab in plaque psoriasis-results of two phase 3 trials. N Engl J Med 2014 Jul 24; 371(4): 326-338.
- Griewank KG, et al: TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J Natl Cancer Inst 2014 Sep 13; 106(9). pii: dju246.
- Lee S, et al: TERT Promoter Mutations Are Predictive of Aggressive Clinical Behavior in Patients with Spitzoid Melanocytic Neoplasms. Sci Rep 2015 Jun 10; 5: 11200.
- Kelleher M, et al: Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predicts and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015 Apr; 135(4): 930-935.e1.
- Simpson E, et al: Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol 2014 Oct; 134(4): 818-823.
- Fuller LC, et al: British Association of Dermatologists’ guidelines for the management of tinea capitis 2014. Br J Dermatol 2014 Sep; 171(3): 454-463.
- Binder B, et al: Tinea capitis in early infancy treated with itraconazole: a pilot study. J Eur Acad Dermatol Venereol 2009 Oct; 23(10): 1161-1163.
- Ramien ML, et al: Quality of life in pediatric patients before and after cosmetic camouflage of visible skin conditions. J Am Acad Dermatol 2014 Nov; 71(5): 935-940.
DERMATOLOGY PRACTICE 2015; 25(4): 39-41