Long-term data from the pivotal DISCOVER-2 study show that treatment with guselkumab resulted in sustained improvements in joint and skin symptoms and functional capacity over a two-year period in biologic-naïve patients with PsA. Evaluations of pooled analyses of two Phase III studies were also extremely promising. Among other things, sustained improvements in enthesitis and dactylitis could also be demonstrated.
The interleukin (IL) inhibitor guselkumab, already established for the treatment of plaque psoriasis, has also been shown to be effective in patients with psoriatic arthritis (PsA), as demonstrated by study data and clinical experience. Guselkumab is a humanized monoclonal antibody that specifically targets the p19 subunit of IL-23, a regulatory cytokine implicated in the pathogenesis of PsA. In Switzerland, guselkumab (Tremfya®) has been approved for the treatment of PsA since February 2021. About 20-30% of all psoriasis patients develop psoriatic arthritis in the course of the disease. During an interdisciplinary panel discussion at this year’s annual meeting of the SGDV, three experts, Prof. Nikhil Yawalkar, Bern, MD, Prof. Jean Dudler, Fribourg, MD, and Timur Taskesen, Zurich, MD, discussed current findings and empirical values from a dermatological and rheumatological perspective [1].
According to the updated EULAR recommendations for the management of PsA, patients should continue to be treated on an individualized basis according to phenotype and predominant symptoms [2]. As before, the following six disease domains are distinguished: peripheral arthritis, skin involvement, nail involvement, axial involvement, dactylitis, and enthesitis.
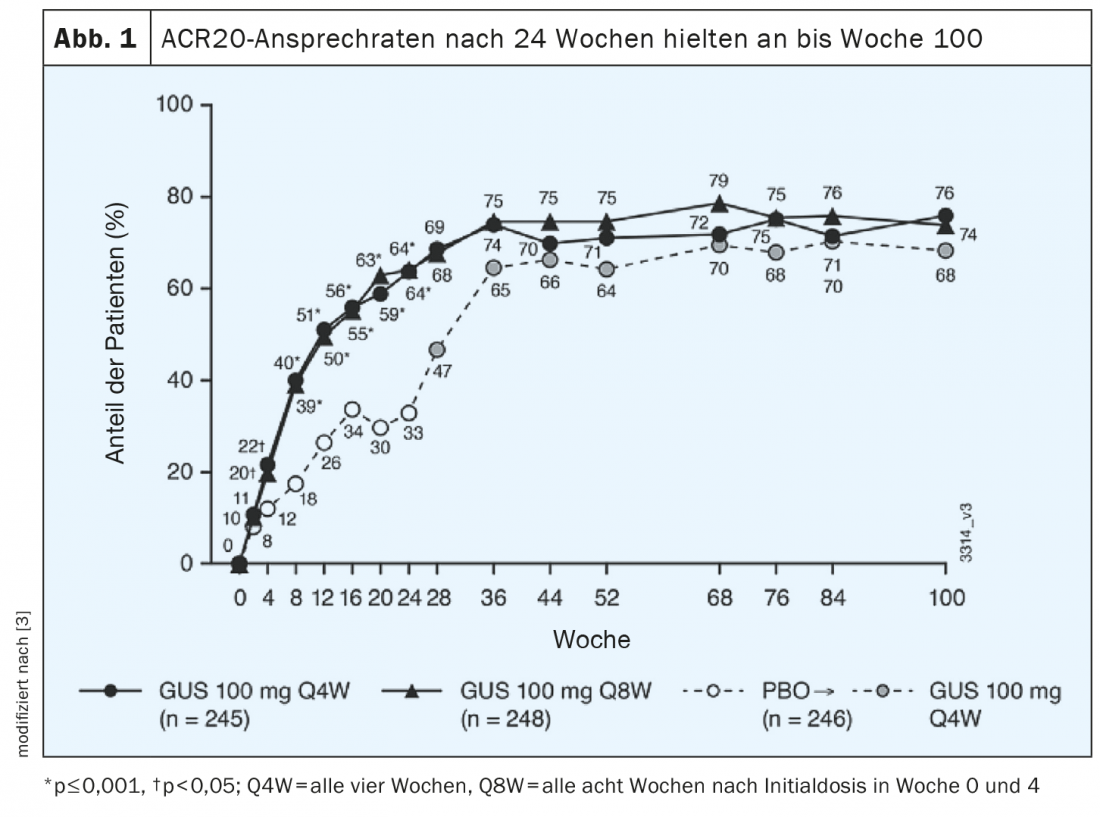
ACR-20 response rates sustained into week 100.
The 2-year data from the phase III DISCOVER-2 trial show that ACR20 response rates achieved with guselkumab at week 24 continued to increase and were maintained through week 100 (Fig. 1) [3]. Of 739 randomized patients, 88% had continued study treatment through week 100; the two verum groups received guselkumab 100 mg every four weeks (q4w) or guselkumab 100 mg every 8 weeks (q8w)*; the control group received placebo every four weeks. At week 100, ACR20 response rates were 76% for q4w and 74% for q8w. Rates of radiographic progression# measured from weeks 52-100 were 0.75 for q4w (n=227) and 0.46 for q8w (n=232). In placebo participants switched to q4w after week 24 (n=228), radiographic progression was 1.12 during weeks 0 to 24 (placebo phase), 0.51 during weeks 24-100 (under q4w), and was 0.13 during weeks 52-100. The safety profile of guselkumab in PsA proved comparable over the 2-year period to that at 6 months and 1 year and was similar in both verum groups (q4w and q8w).
* q4w = every four weeks, q8w = every eight weeks after initial dose at weeks 0 and 4.
# using PsA-modified radiographic vdH-S scores
Pooled analysis: improvements in different target domains
A post hoc analysis based on data from the DISCOVER-1 and DISCOVER-2 trials was published in a June issue of Lancet Rheumatology this year [4]. Patients with PsA and axial involvement with a radiological finding of sacroiliitis were included. Of the 312 randomized study participants (61% male, mean age, 45.1 ± 11.2 years) with PsA and sacroiliitis, 103 patients received guselkumab 100 mg every four weeks (q4w) and 91 subjects were treated with guselkumab 100 mg every 8 weeks (q8w)*. There were 118 study participants assigned to the placebo group. At weeks 8 and 16, improvements in Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Ankylosing Spondylitis Activity Score (ASDAS) scores were greater in the two guselkumab groups compared with placebo (both p<0.01). Least-square means (LSM)** at week 24 showed that patients on guselkumab performed better in BASDAI compared with placebo (difference of -1.3 [95% CI, -1.9 to -0.7] for both guselkumab groups).
* q4w = every four weeks, q8w = every eight weeks after initial dose at weeks 0 and 4.
** LSM (“Least-square-means”) = mean values for which the influence of covariates on the dependent variable was removed.
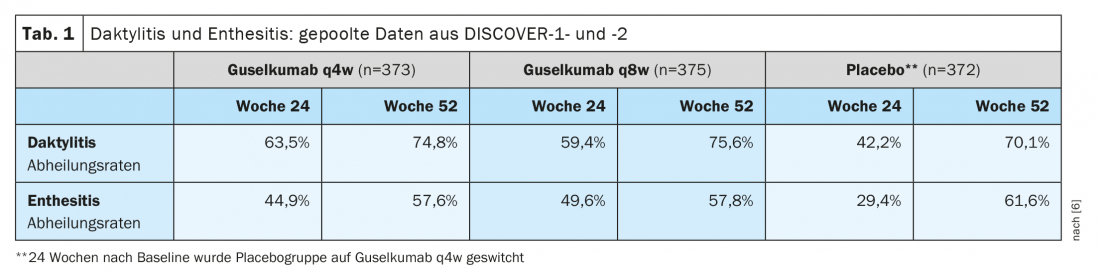
Further pooled analysis of data from DISCOVER-1 and DISCOVER-2 showed that in patients with dactylitis or enthesitis at baseline, the healing rates achieved at week 24 were maintained or even improved by week 52 (Table 1) [6]. This is an important finding, as enthesitis and dactylitis are associated with higher disease activity and more severe pain, and overall can significantly affect the quality of life of affected individuals [5].
Congress: SGDV Annual Conference 2021
Literature:
- Satellite Symposium 16: “IL-23 inhibitors for a holistic patient care – indisciplinary panel discussion Guselkumab – a treatment for everyone?”, SGDV Annual Meeting, 25-27 Aug 2021.
- Coates LC, et al: The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) Treatment Recommendations 2021, eEULAR 2021, Abstract OP0229.
- McInnes IB, et al: Efficacy and Safety of Guselkumab, a Monoclonal Antibody Specific to the p19-Subunit of Interleukin-23, Through 2 Years: Results from a Phase 3, Randomized, Double-blind, Placebo-controlled Study Conducted in Biologic-naïve Patients with Active Psoriatic Arthritis. Abstract 50, Innovations in Dermatology 2021.
- Mease PJ, et al: Efficacy of guselkumab on axial involvement in patients with active psoriatic arthritis and sacroiliitis: a post-hoc analysis of the phase 3 DISCOVER-1 and DISCOVER-2 studies. Lancet Rheumatol. Published online June 29, 2021. doi:10.1016/S2665-9913(21)00105-3.
- Polachek A, et al: Clinical enthesitis in a Prospective Longitudinal Psoriatic Arthritis Cohort: Incidence, Prevalence, Characteristics, and Outcome. Arthritis Care Res (Hoboken) 2017; 69(11): 1685-1691.
- McInnes IB, et al: Efficacy and Safety of Guselkumab, an Interleukin-23p19-Specific Monoclonal Antibody, Through One Year in Biologic-Naive Patients With Psoriatic Arthritis. Arthritis & Rheumatology 2021; 73(4): 604-616.
DERMATOLOGIE PRAXIS 2021; 31(6): 22-24
InFo PAIN & GERIATry 2021; 3(2): 21-22.