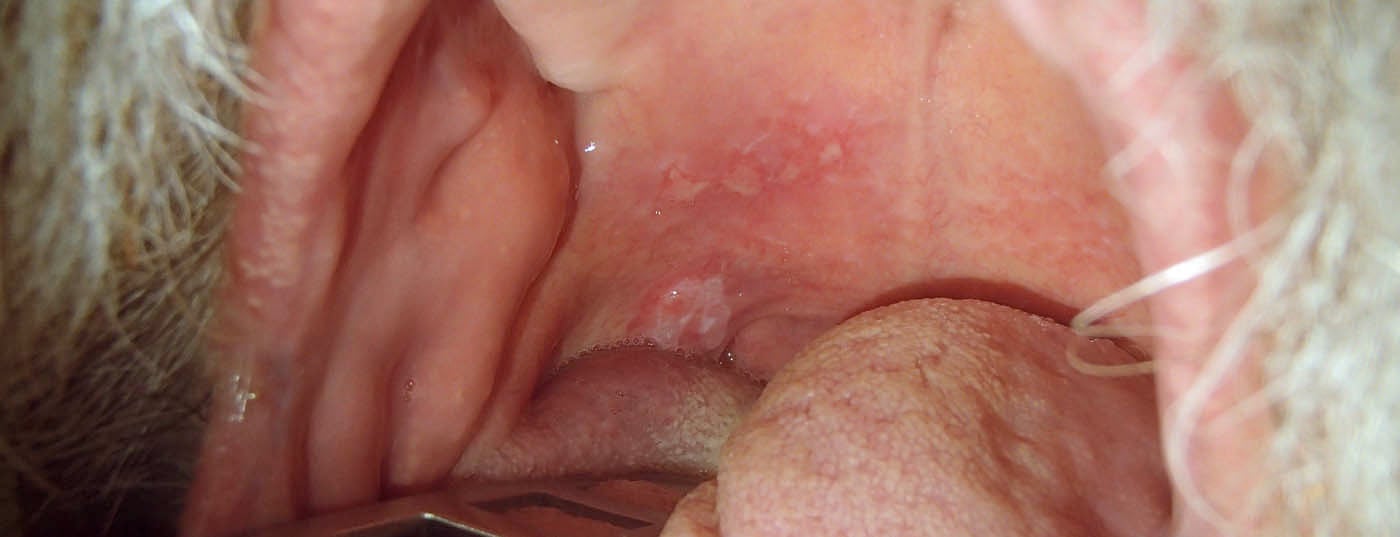
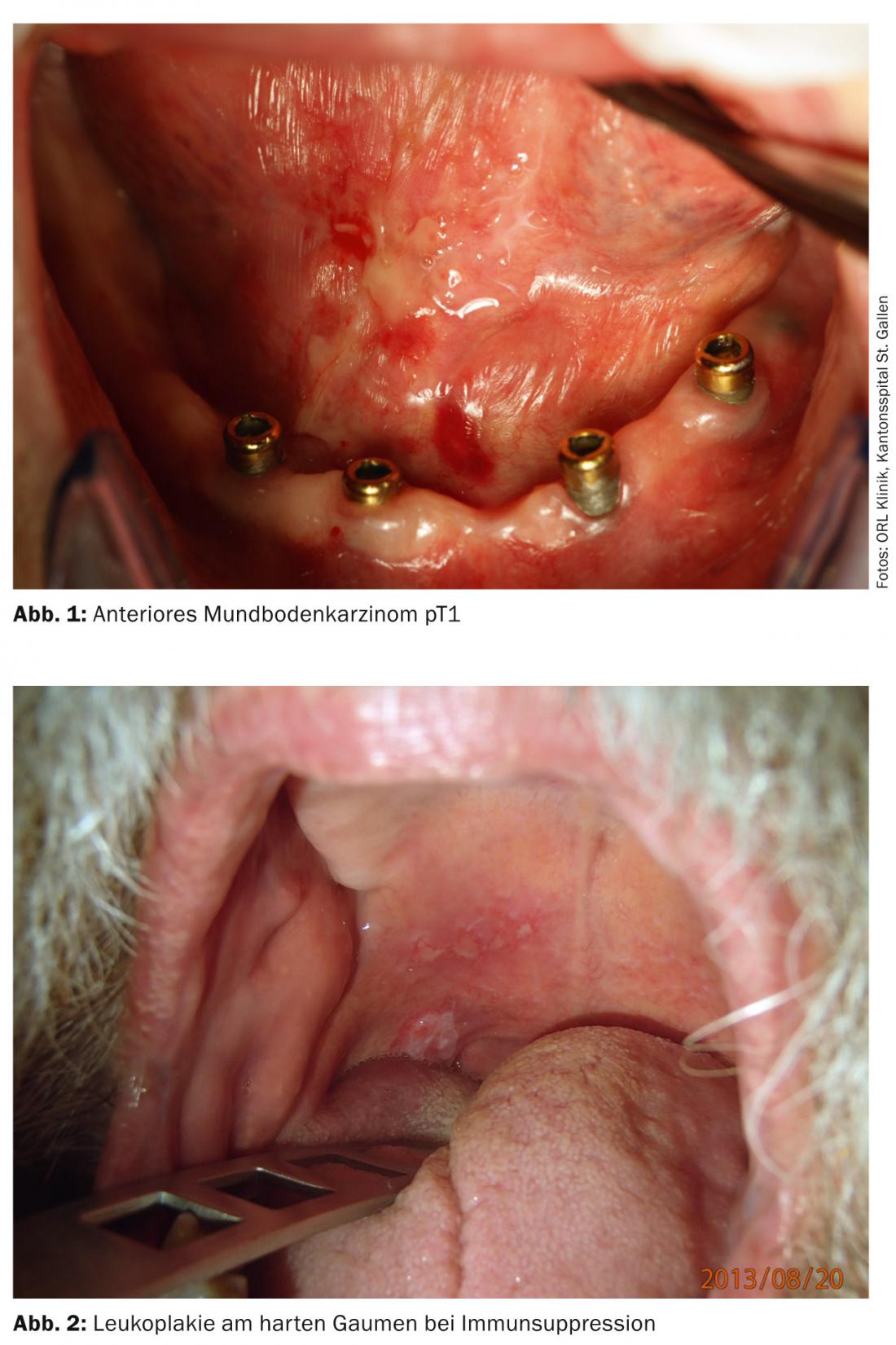
Important risk factors for oral cavity carcinomas in our latitudes are tobacco and alcohol use, chronic infection with high-risk HPV, immunosuppression, and previous tumors in the head and neck region. Often, the carcinomas are not detected until the late stages; therefore, the overall survival rate of patients with oral cavity carcinoma is only 50%. Treatment consists primarily of surgical tumor resection with or without adjuvant radio(chemo)therapy. In all patients with T1/T2cN0 stages, sentinel lymph node biopsy is recommended to determine the need for neck dissection.
Approximately 5% of all malignant tumors worldwide are oral cavity carcinomas, 95% of which are squamous cell carcinomas [1]. The peak age is 60 years. There is a slight predisposition to the male sex.
Risk factors: smoking, alcohol, and infection with high-risk HPV.
Main risk factors for carcinoma development in the oral cavity are tobacco use (more than 1 pack/d) and alcohol abuse (more than 100 g/d). In other countries such as India, where betel nut and chewing tobacco are consumed, oral cavity carcinoma is even one of the most common malignancies. Another cause is immunosuppression, which has become more important with increasing numbers of immunosuppressed patients, for example after organ transplantation. The incidence rate in individuals after transplantation is 5% after 20 years and depends mainly on the degree of graft-versus-host reaction [2].
In recent years, there has been an increase in indications in younger patients under 45 years of age, which may be explained by an increasing prevalence of carcinomas caused by high-risk types of human papillomavirus (HPV), analogous to oropharyngeal carcinoma. However, the proportion of HPV-associated carcinomas is significantly lower in the oral cavity (less than 20%) than in the oropharynx (more than 50%).
Another important risk factor for the development of a malignant tumor in the upper airway is a previous malignant tumor in the head and neck region. In a retrospective evaluation of nearly 100,000 patients, the cumulative risk after 20 years was 36% of developing a second primary tumor in the upper aerodigestive tract [3]. The reason for this could be the data provided by Slaughter et al. first described in 1953, which describes that carcinogenic effects of tobacco and alcohol act simultaneously on multiple areas of the upper aerodigestive tract and trigger the development of multiple, independent primary tumors [4].
Precancerous lesions
Some oral cavity carcinomas arise because of a precursor lesion or premalignant conditions. Leukoplakia, which occurs with a prevalence of 0.2-5%, is considered an optional precancerous condition; obligatory precancerous conditions are erythroplakia and verrucous leukoplakia. Leukoplakia may correspond histopathologically to simple epithelial hyperplasia to invasive carcinoma [5]. Lesions on the floor of the mouth and the tongue margin are more likely to have dysplastic areas compared with buccal mucosa [6].
Since the differentiation between a benign, premalignant or malignant change is hardly possible clinically, biopsy collection is recommended. Mild and moderate dysplasias should be followed in their course, as 3-8% of leukoplakias degenerate over a five-year period. If it is a high-grade dysplasia histopathologically, the lesion is removed with a safety margin of 0.5 cm. Because of the risk for a false-negative finding, especially with large-area changes, mucosectomy is recommended as an alternative [5]. In case of erythroplakia as obligate precancerous lesions, surgical resection is recommended in any case.
Methods for early diagnosis
Tumors of the upper air and food passages often remain asymptomatic for a long time and are thus discovered late (Fig. 1 and 2) . Thus, only one third of patients with oral cavity carcinoma are diagnosed at an early stage without regional metastasis. Approximately 10% of patients already have distant metastases at diagnosis [7].
Because the timing of diagnosis directly affects a patient’s prognosis, early detection of a premalignant or invasive lesion is of great importance. To improve early diagnosis, staining of tissue with dyes such as toluidine blue was propagated; in addition, various technical methods such as autofluorescence diagnostics, optical spectroscopy, optical coherence tomography or narrow band imaging (NBI) were developed. In a meta-analysis examining the use of toluidine blue in the head and neck region, the sensitivity for detection of invasive tumor was 78-100%, and the specificity was 30-100%. In dysplasia, the method was positive in only about half of the cases [8]. The technique has not gained acceptance as a screening method because of its low specificity [9,10], as also shown in a recent meta-analysis. This compared toluidine blue staining, cytodiagnostics, and diffuse reflectance spectroscopy (DRS) with laser-induced autofluorescence (LIAF) [11]. The highest diagnostic accuracy was achieved by the DRS (97%) and the LIAF (96%). Both techniques were clearly superior to the other methods, with toluidine blue staining performing the worst (67%).
Volgger et al. investigated the significance of optical coherence tomography for tumor diagnosis in the upper aerodigestive tract [5]. The authors conclude that optical coherence tomography is a suitable tool for the early diagnosis of tumors due to its high resolution and ease of use, but it cannot replace a biopsy. A similar conclusion was reached in previous studies [12,13].
Narrow band imaging (NBI) is focused on image contrast enhancement of superficial vascular structures, revealing characteristic vascular neoplasms in premalignant or invasive lesions that can be used for early diagnosis. A recent review showed a diagnostic accuracy of NBI of 92-97% compared with 66-89% for white light endoscopy; thus, NBI has potentially great utility for the detection and evaluation of premalignant and invasive lesions [14]. In principle, however, early histological clarification of changes in the oral mucosa remains the diagnostic method of choice.
Staging
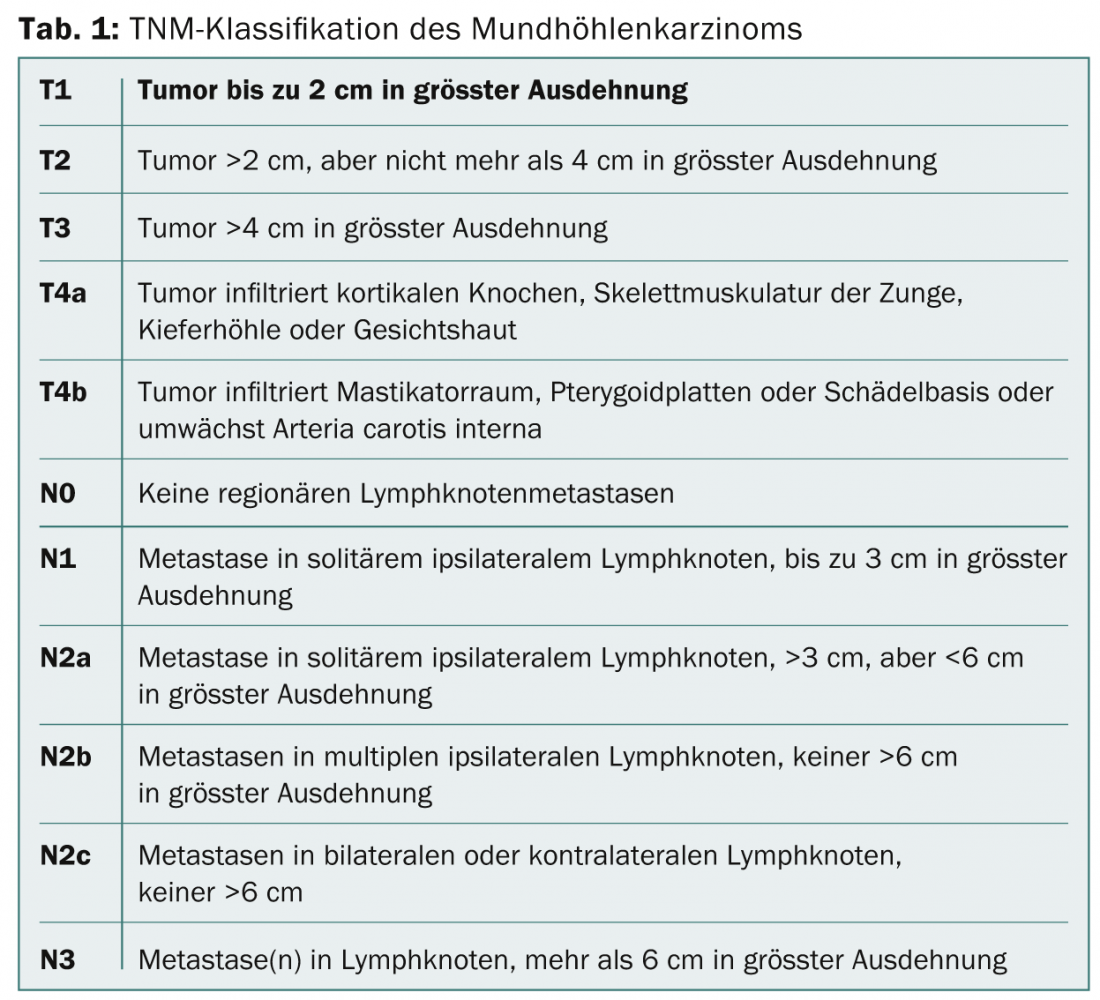
In the presence of oral cavity carcinoma, the therapeutic decision should be made after discussion at an interdisciplinary tumor board, taking into account the exact location of the tumor, tumor stage according to TNM classification (Tab. 1), concomitant diseases, and the patient’s wishes. If the biopsy has confirmed the diagnosis of oral cavity carcinoma, sonography of the soft tissues of the neck with fine needle aspiration of the lymph nodes must be performed for staging. Radiological clarification of the primary tumor is preferably performed by magnetic resonance imaging (MRI), especially since MRI can better visualize soft tissue extension and has the same sensitivity regarding bone infiltration into the mandible or maxilla as high-resolution computed tomography (CT) [15]. Usually, upper panendoscopy is performed under anesthesia for detailed clinical evaluation of the tumor and to exclude synchronous second tumors, which can be detected in about 5% of patients [16]. In advanced tumor stages (cT3-4 or cN2-3), positron emission tomography (PET) with fluorodeoxyglusoe (FDG) has become established for staging and exclusion of distant metastases [17].
Therapy options
For the treatment of early tumor stages (cT1-2 and cN0-1), “single-modality therapy” is usually sufficient, usually in the form of surgery alone [15]. Alternatively, radiotherapy (percutaneous radiation or brachytherapy) may be considered. Advanced oral cavity carcinomas (cT3-4, >cN1) are usually treated multimodally with surgical resection and adjuvant radiotherapy with or without concomitant chemotherapy [15]. Based on the work of Bernier and Cooper, the indication for adjuvant chemotherapy with cisplatin to adjuvant radiation is given for extracapsular tumor growth (ECS), lymphangiosis carcinomatosa, perineural tumor spread, and positive resection margins [18–20]. A recent study confirms that, especially in patients with ECS, 2- and 5-year survival can be significantly improved by chemotherapy [21].
Primary surgery followed by adjuvant radiotherapy with or without chemotherapy is the treatment of choice for advanced carcinoma in most centers. Cohen et al. but show comparable functional outcomes and survival rates in primary irradiated patients with T4 carcinomas [22]. In contrast, the study by Gore et al. a clear survival advantage of patients treated primarily by surgery [23].
Radiotherapy carries the risk of osteonecrosis occurring as a long-term consequence, especially of the mandible [24]. The incidence is 2-22% [25]. However, osteonecrosis rarely occurs after irradiation with a dose of less than 60 Gy. Hyperfractionated or moderately accelerated radiation and the use of intensity-modulated radiotherapy appear to minimize the risk [26,27]. Brachytherapy and concomitant chemotherapy, on the other hand, are risk factors for osteonecrosis [24,28,29].
Reconstruction challenges
The challenge in surgery is to resect the tumor with sufficient safety margin and still preserve function. Posttherapeutic deficits in swallowing and speech function are common and significantly affect quality of life [30]. According to recent surveys, 50-75% of long-term survivors are affected by some degree of swallowing and/or articulation disorders [31–33].
Because the location and extent of resection correlate with postoperative dysfunction, the reconstruction technique chosen should consider both the anatomic aspect and function [34]. Various techniques have been developed for this purpose. They range from primary closure, healing per secundam, split skin grafts, displacement flaps, and pedicled flaps to free microvascular anastomosed flaps. The management of resection defects varies widely and depends in no small part on the experience of the surgeon. According to Shah et al. superficial defects of the mucosa and underlying soft tissues can be adequately closed with split skin grafts [35]. Our own data show that in early stages, reconstruction of the defect is often unnecessary and healing per secundam yields good functional results [36].
Management of the lymphatic drainage area
20-30% of patients with early-stage tumors (T1/T2cN0) have occult metastases, which is why elective neck dissection is preferred over a “watch-and-wait” strategy [37]. The extent of neck surgery has changed over the past decade from radical procedures to minimally invasive techniques. As an alternative to elective neck dissection, sentinel lymph node biopsy (SNB) has become established at many centers over the past 15 years for all patients with T1/T2cN0. Positive lymph nodes are detected in up to 38% of patients and a completion neck dissection is performed. If extracapsular tumor growth is evident or more than two lymph nodes are involved, adjuvant radiochemotherapy is indicated [38]. SNB is associated with decreased surgical risk and significantly lower morbidity than elective neck dissection [39,40]. In patients with cytologically verified or radiologically probable lymph node metastases, there is an indication for simultaneous resection of the primary tumor and therapeutic neck dissection.
Prognostic factors
The overall survival rate of patients with oral cavity carcinoma is 50%; this rate has not increased significantly in recent decades despite technical advances in therapy and imaging [15]. In addition to tumor stage, several histopathologic parameters have emerged as important prognostic factors. According to one of our studies, however, it is not the tumor thickness and infiltration depth but the tumor differentiation grade and a dissolute growth pattern at the tumor front that seem to significantly increase the risk for lymphogenic metastasis and thus influence survival [41].
Resection with an adequate margin of safety is of critical prognostic importance; however, the definition of adequate resection margins always provides a source of debate. In 1978, positive resection margins were reported by Looser et al. defined as a distance to the tumor of less than 5 mm or dysplastic cells in the resection margin [42]. This concept is still in use. It requires resection with a surgical resection margin of at least 1 cm due to tumor shrinkage in formalin, which is not always possible depending on the size and location of the tumor.
Future concepts are expected to give higher weight to the importance of molecular alterations in the resected margins [43]: The epigenetic alterations of tumor cells, which are crucial for tumorigenesis and are increasingly identified, are to be determined intraoperatively with quantitative polymerase chain reactions (PCR) in a rapid procedure [44]. This allows the necessary resection margins to be defined, and these factors can also be taken into account when determining the indication for adjuvant therapy. Supic et al. investigated aberrant hypermethylation of several genes (p16, DAPK, E-cad and others) and found that in histologically negative resection margins, hypermethylation of the DAPK gene in particular was associated with poorer survival [45]. In our opinion, the longer this principle is applied, the more it will find its way into clinical routine.
Take-home messages for the practitioner
- 95% of oral cavity carcinomas are squamous cell carcinomas.
- They are often only recognized in the late stages.
- Oral cavity carcinomas are primarily treated surgically with or without adjuvant radio(chemo)therapy.
- For T1/T2cN0 stages, sentinel lymph node biopsy is recommended.
- In the future, the determination of molecular alterations of the resectate margins will gain importance for adequate surgical resection.
Martina A. Broglie Däppen, MD
Séverine M. Niederer-Wüst, M.D.
Prof. Dr. med. Sandro J. Stöckli
Literature:
- Thomas L, et al: Am J Otolaryngol 2012; 33(6): 650-656.
- Curtis RE, et al: Blood 2005; 105(10): 3802-3811.
- Chuang SC, et al: Int J Cancer 2008; 123(10): 2390-2396.
- Slaughter DP, et al: Cancer 1953; 6(5): 963-968.
- Volgger V, et al: Head Neck 2013; 35(11): 1558-1566.
- Neville BW, Day TA: CA Cancer J Clin 2002; 52(4): 195-215.
- Howlader N, et al: J Natl Cancer Inst 2009; 101(7): 533-36.
- Gray M, et al: Br Dent J 2000; 189(3): 125.
- Lingen MW, et al: Oral Onc 2008; 44(1): 10-22.
- Patton LL, et al: J Am Dent Assoc 2008; 139(7): 896-905.
- Fuller C, et al: Head Neck 2014 Mar 5. [Epub ahead of print]
- Wilder-Smith P, et al: Lasers Surg Med 2009; 41(5): 353-357.
- Hamdoon Z, et al: Photodiagnosis Photodyn Ther 2011; 8(1): 49-52.
- Vu AN, Farah CS: Oral Oncol 2014; 50(5): 413-420.
- Belcher R, et al: J Surg Oncol 2014; 110(5): 551-574.
- Haerle SK, et al: Head Neck 2010; 32(3): 319-325.
- Arias F, et al: Clin Transl Onc 2014 Jul 31. [Epub ahead of print]
- Bernier J, et al: N Engl J Med 2004; 350(19): 1945-1952.
- Bernier J, Cooper JS: Oncologist 2005; 10(3): 215-224.
- Cooper JS, et al: NEJM 2004; 350(19): 1937-1944.
- Zhang H, et al: J Otolaryngol Head Neck Surg 2013; 42: 30.
- Cohen EE, et al: Head Neck 2009; 31(8): 1013-1021.
- Gore SM, et al: Head Neck. 2014 Feb 15. [Epub ahead of print]
- Schwartz HC, et al: Am J Clin Onc 2002; 25(2): 168-171.
- Store G, et al: Clin Otolaryngol Allied Sci 2000; 25(5): 378-384.
- Lyons A, Ghazali N: Br J Oral Maxillofac Surg 2008; 46(8): 653-660.
- Studer G, et al: Radiation Onc 2006; 182(5): 283-288.
- Kuhnt T, et al: Med Oncol 2006; 23(3): 325-333.
- Hehr T, et al: Radiother Oncol 2006; 80(1): 33-38.
- Dwivedi RC, et al: Eur Arch Otorhinolaryngol 2012; 269(4): 1233-1239.
- Suarez-Cunqueiro MM, et al: Arch Otolaryngol Head Neck Surg 2008; 134(12): 1299-1304.
- Pauloski BR, et al: Head Neck 2006; 28(12): 1069-1076.
- Gillespie MB, et al: Arch Otolaryngol Head Neck Surg 2005; 131(7): 615-619.
- de Bree R, et al: Eur Arch Otorhinolaryngol 2008; 265(1): 1-9.
- Shah JP, Gil Z: Oral Oncol 2009; 45(4-5): 394-401.
- Romer, data not yet published.
- Stoeckli SJ, Broglie MA: Curr Opin Otolaryngol Head Neck Surg 2012; 20(2): 103-08.
- Broglie MA, et al: Head Neck 2013; 35(5): 660-666.
- Schiefke F, et al: Head Neck 2009; 31(4): 503-512.
- Murer K, et al: Head Neck 2011; 33(9): 1260-1264.
- Goerkem M, et al: Ann Surg Onc 2010; 17(2): 527-535.
- Looser KG, et al: Head Neck Surg 1978; 1(2): 107-111.
- Weinstock YE, et al: Oral Maxillofac Surg Clin North Am 2014; 26(2): 151-162.
- Goldenberg D, et al: Arch Otolaryngol Head Neck Surg 2004; 130(1): 39-44.
- Supic G, et al: Oral Oncol 2011; 47(8): 702-708.
InFo ONCOLOGY & HEMATOLOGY 2014; 2(10): 6-10.