Although the symptoms of acute heart failure can be treated successfully for the most part, the long-term prognosis remains poor. This could change, at least for some patients, as a study with the active ingredient Serelaxin shows.
The RELAX-AHF trial investigated the effect of serelaxin in patients with acute heart failure [1]. Serelaxin is the recombinant form of the human peptide hormone relaxin-2, which is produced more abundantly during pregnancy and mediates various hemodynamic adaptations.
These include increases in cardiac ejection fraction, decreases in systemic vascular resistance, and increased renal blood flow. Relaxin-2 also appears to possess anti-ischemic, anti-inflammatory, and anti-fibrotic properties.
The multicenter, double-blind, placebo-controlled RELAX-AHF trial included 1150 patients hospitalized for acute heart failure. With the aim of minimizing the development of end-organ damage by early initiation of therapy, subjects were administered placebo or serelaxin 30 μg/kg/day i.v. for 48 hours in addition to standard therapy within 16 hours of admission. Primary endpoints of the study were first, the decrease in dyspnea after five days as measured by the visual analog scale (VAS), and second, the proportion of patients who reported moderate or marked improvement in their dyspnea on the Likert scale during the first 24 hours. Secondary end points were defined as days of survival and days out of hospital, and the number of cardiovascular deaths and re-hospitalizations 60 days after treatment. In addition, all-cause mortality was recorded after 180 days (safety endpoint).
Results of the RELAX-AF study
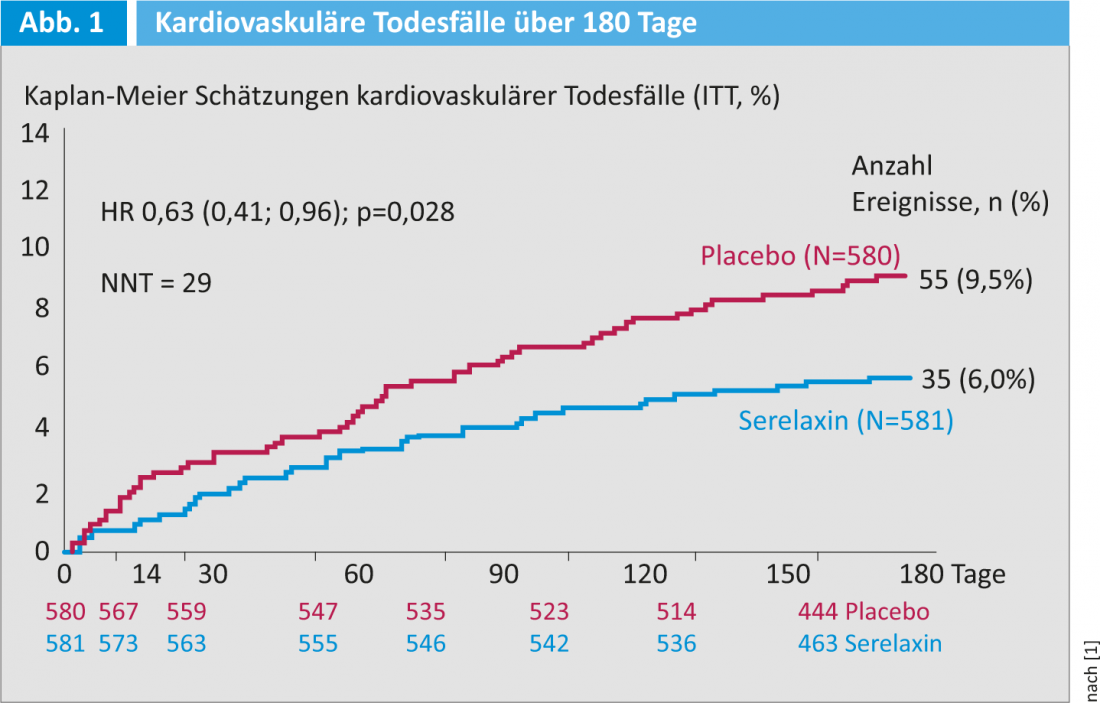
As the results of the study showed, dyspnea had significantly improved in the Serelaxin group compared to baseline at five days (VAS). No difference to placebo was observed regarding the other endpoints. The result of the six-month follow-up was all the more surprising. This showed that 37% fewer deaths occurred in the Serelaxin group than with placebo (42 vs. 65, HR 0.63 [95% CI 0.43-0.93; p=0.02]). An equally large reduction was seen in cardiovascular-related deaths (Fig. 1) . “The significance of this result is limited by the fact that the study was not planned to investigate mortality,” said Prof. Piotr Ponikowski, MD. For the first time, however, it has been possible to show that mortality can be influenced by early intervention.
Paradigparadigm shift in heart failure therapy is called for.
The study results are consistent with Prof. Ponikowski’s call for a paradigm shift in acute heart failure therapy. Current treatment focuses primarily on rapid symptom relief. “What we need is a treatment that works quickly while improving the long-term prognosis,” Prof. Ponikowski said. For the study population under investigation, which includes elderly patients with normal to elevated blood pressure and various comorbidities, Serelaxin may represent such a treatment. “However, to believe that the different entities of acute heart failure can be treated with only one therapy is naive.”
Source: Cardiology Update Davos 2013,February 10-15.
Literature:
- Teerlink JR, et al: Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 2013; 381(9860): 29-39.