Selection of the appropriate therapy and the optimal fitting wound dressings are decisive factors in the healing process. Consideration of the individual wound microenvironment plays an important role, as recent findings show.
In contrast to other specialties in medicine, there is a relatively small evidence base in wound research, which should change in the future. Synergies between clinical practice and scientific research are particularly important in this area. Prof. Dr. med. Ewa K. Stürmer, Director of the Institute for Translational Wound Research at the University of Witten/Herdecke (D) [1], spoke about current findings in this field at the Nuremberg Wound Congress 2019.
Biofilm: what is possible in the outpatient setting?
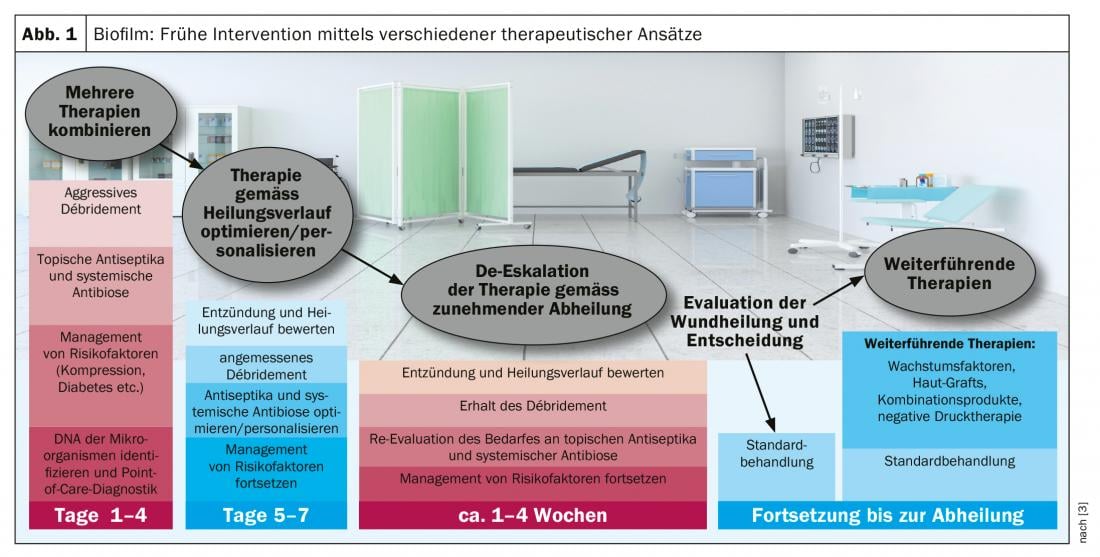
Biofilm (box) forms in 78% of all chronic wounds with potentially negative effects on wound healing [2]. That the wound environment is a key factor in antimicrobial performance in the context of biofilm therapy is one of the current findings in this field [2]. Only mechanical debridement can fully clean a biofilm. However, outpatient wound care can reduce the microbial load, which prevents biofilm from developing into infection (Fig. 1) . Proven methods for this are: intensive wiping and NaCl, wound irrigation solutions, spray disinfection, antiseptic wound dressing. In general, the following principles apply to the care of the wound edge and environment: antimicrobial substances tend to have an anti-proliferative effect; edema should be taken into account, skin moisture should be ensured, and basic care should be provided. A biofilm does not per se cause an infection and can also be on a wound that does not look infected at all. The wound margin is often affected by biofilm. This can be visualized by means of fluorescence measurement, since bacteria have an intrinsic fluorescence and when irradiated with UV light bacterial colonization is stained at a concentration >104/m2. The speaker illustrates this with the case study of a diabetic foot ulcer. “For eliminating biofilm, the best and only therapy of choice at the moment is mechanical removal by curette or scalpel” [1]. This is also the conclusion of current consensus recommendations [3].
Hydrophobic wound meshes and smart wound dressings
“Hydrophobic wound meshes are not only cost-saving but also cell-protective” [1]. For a locally infected wound, the speaker recommends switching to a hydrophobic wound mesh after 7-10 days of antimicrobial dressings and wound irrigation solutions. The mechanism of action is due, among other things, to the structure of this wound network, which favorably influences bacterial uptake.
The topic of smart wound technologies is a controversial one. On the one hand, the industry already offers corresponding products by means of which, for example, ph-value, temperature and hypoxia can be measured. On the other hand, what to do based on this information is another dimension. The speaker points out that there is still a large gap in this regard. Recognizing for which wound, which treatment is indicated and which materials and methods are conducive to the healing process or not, is a central question, the processing of which takes place on another level. Perhaps there will also be further synergies between these areas in the future.
| Biofilm Biofilm is a structured community of microbes with genetic diversity and variable gene expression that creates behaviors and defenses leading to the generation of unique (chronic) infections with significant tolerance to antibiotics and antimicrobials while protecting against host immunity [1]. |
Place of silver in antiseptic therapy
Silver is included in numerous wound care products and according to a recent meta-analysis [4], when used in a targeted and time-limited manner, there is evidence of an improvement in quality of life as well as good cost-effectiveness, in addition to antimicrobial effects. Correct indication and therapy duration over a maximum period of 14 days are crucial [4]. Clinically relevant silver resistance could not be demonstrated [5]. The conclusion of a 2019 article on this is that there is no evidence at this time that silver resistance is a serious health threat [5]. Further studies are needed to make clearer statements on this and to determine the amount of silver required for an optimal therapeutic effect.
Source: Wound Congress Nuremberg (D)
Literature:
- Stürmer EK: Wound colonization, infection, or allergy? How to recognize and treat? Prof. Dr. med. Ewa K. Stürmer, Institute for Translational Wound Research, University of Witten/Herdecke (D), slide presentation, Nuremberg Wound Congress 06.12.2019.
- Hülsbömer LF, Rembe JD, Besser M, Stürmer EK: Quantitative and qualitative evaluation of the anti-biofilm efficacy of modern antimicrobial and antiseptic agents in a new bacterial in vitro biofilm model (hpBIOM). Contribution FV37, Nuremberg Wound Congress, Dec 07, 2019.
- Schultz G, et al: (for the Global Wound Biofilm Expert Panel): Consensus guidelines for the identification and treatment of biofilms in chronic nonhealing wounds. Wound Repair and Regeneration 2017; 25 (5): 744-757.
- Dissemond J, et al: Evidence for silver in wound care – meta-analysis of clinical studies from 2000-2015. J Dtsch Dermatol Ges 2017; 15(5): 524-535.
- Percival SL, Salisbury AM, Chen R: Silver, biofilms and wounds: resistance revisited. Crit Rev Microbiol 2019; 45(2): 223-237.
HAUSARZT PRAXIS 2020; 15(1): 28-30 (published 1/25/20, ahead of print).