The spectrum of systemic agents approved for the treatment of moderate to severe atopic dermatitis has recently expanded, so that there is now a choice of several biologics and Janus kinase inhibitors. Itch relief is a key treatment goal for sufferers – what is known about the antipruritic effects of the new systems therapeutics? This question is discussed in a recent mini-review published in the Journal of the Dermatological Society.
The first author of the publication is Hanna Bonnekoh, MD, from the Department of Dermatology, Venereology and Allergology, Charité Universitätsmedizin Berlin [1]. The importance of knowledge about the effects of new drugs on the improvement of pruritus is shown, among others, by a recently published survey of 1104 patients with atopic dermatitis: pruritus was considered very important in assessing the efficacy of a treatment by 95.4% of the patients [2]. In clinical trials of atopic dermatitis, itch parameters are usually reported using standardized endpoints, making it possible to compare active ingredients with respect to their effects on itch.
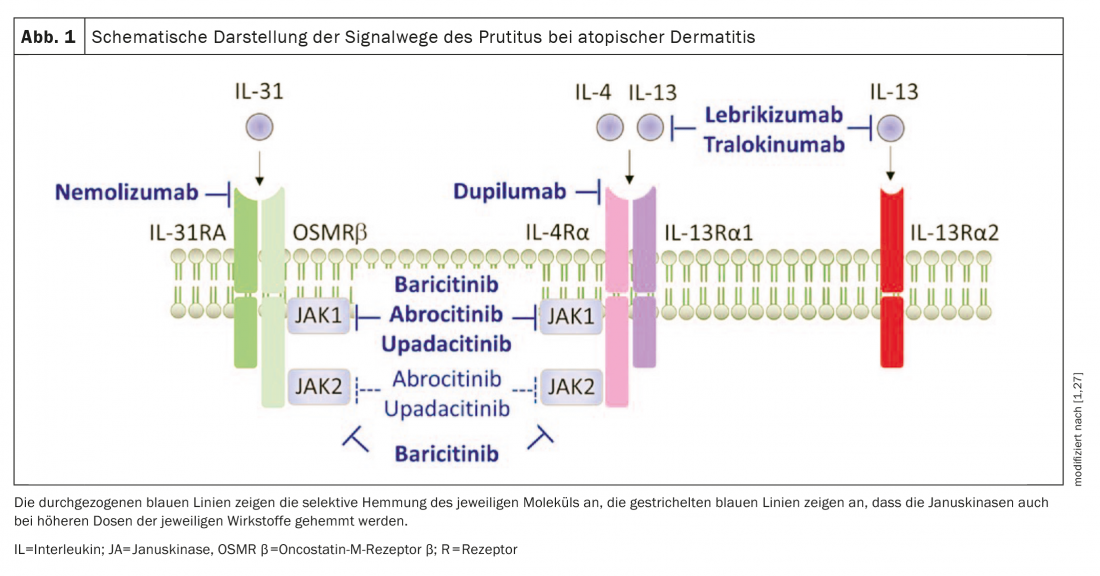
Overall, all of the studies included in the review by Bonnekoh et al. 2022 new drugs in atopic dermatitis (Fig. 1) shown promising data in improving itch.
What is the current state of knowledge?
The suffering of those affected is high; among other things, many complain of itch-related sleep disturbances [11–13]. In addition to a rapid onset of action, overall itch reduction over the entire treatment period is also of great importance for atopic dermatitis patients, as shown by Bonnekoh et al. emphasize [1]. The following is a summary of the modern systemic therapies currently approved by the EMA and Swissmedic for the treatment of moderate to severe atopic dermatitis.

Dupilumab: The fully human immunoglobulin (Ig)-G4κ monoclonal antibody Dupilumab (Dupixent®), inhibits IL-4 and IL-13 signaling via inhibition of IL-4Rα. A recent analysis of four randomized phase III clinical trials analyzed the effects of dupilumab on pruritus in patients with moderate to severe atopic dermatitis. This showed that treatment with dupilumab resulted in a rapid and statistically significant improvement in the daily most severe itch (percent change with respect to numerical rating scale, NRS) compared with placebo, as early as day 2 in adults and day 5 in adolescent patients. In addition, rapid improvement (defined as patient proportion with at least 3-point improvement from baseline) in itch was observed from day 4 in adults and from day 13 in adolescents. Furthermore, over the course of the respective studies, itch intensity significantly reduced in all studies compared to placebo and showed a progressive decrease over time [3].
Tralokinumab: The recombinant human IgG4λ monoclonal antibody tralokinumab (Adtralza®) directed against IL-13 prevents IL-13 from binding to IL-13Rα1 and IL-13Rα2 and was recently approved in Switzerland for adults with moderate to severe atopic dermatitis [34]. Results from phase III studies of tralokinumab as monotherapy (ECZETRA1 and 2) and in combination with topical corticosteroid treatment (ECZETRA 3) in patients with moderate to severe atopic dermatitis were very convincing. At week 16, a higher proportion of tralokinumab-treated patients experienced a reduction in weekly average daily most severe pruritus (NRS ≥4) compared with baseline in the placebo comparison. In addition, treatment with tralokinumab showed greater changes from baseline in the daily most severe pruritus NRS compared with the placebo group, with significant difference from treatment week 1 [4,5].
Baricitinib: The oral JAK inhibitor baricitinib (Olumiant®) has equally high specificity for JAK-1 and -2 and lower specificity for Tyk-2 [21,34]. In combination with topical
corticosteroids (TCS), a significant reduction in pruritus (mean NRS compared with baseline) was achieved as early as day 2 with daily treatment with 2 mg or 4 mg baricitinib. At week 16, a significantly higher proportion of patients in the treatment arm (44% in the 4-mg group, 38% in the 2-mg group) showed clinically significant improvement in itch (defined as change from baseline NRS ≥4) compared with the placebo group (20%) [6,7].
Upadacitinib: Upadacitinib (Rinvoq®), another small molecule drug, is an oral inhibitor of JAK-1 [8,9,34]. Monotherapy with 15 mg or 30 mg (1×/d each) resulted in a significant improvement in pruritus at week 16 compared with placebo, as well as a significant reduction in pruritus from baseline (NRS ≥4) [10]. Also combined with TCS, upadacitinib was convincing in a phase III study.
|
Pathogenesis of pruritus remains a mystery To date, the exact underlying mechanism of pruritus in atopic dermatitis (AD) has not been fully elucidated. Recent evidence suggests that histamine-independent pruritogens are involved in the development of chronic itch [28,29]. In addition to interleukin-31-a cytokine produced predominantly by Th2 cells that causes pruritus via a receptor complex expressed on C fibers-IL-13 and IL-4 are Th2-specific cytokines thought to be involved in the pathogenesis of AD and pruritus symptomatology. Other pruritogens associated with itch in AD, besides histamine, include tryptase, IL-33, and thymic stromal lymphopoietin (TSLP)-but their clinical relevance in triggering itch in AD is still unclear [30]. |
Further active ingredients are in the pipeline
Bonnekoh et al. 2022 also address systemic agents that were still in development at the time of their review. In addition to the now approved JAK inhibitor abrocitinib, the monoclonal antibodies lebrikizumab and nemolizumab are described in terms of their effects on pruritus characteristics [1,34]. The authors note that their review does not discuss novel topical treatment approaches such as the tyrosine kinase 2 (TYK2) inhibitor brepocitinib or the aryl hydrocarbon receptor-modulating agent tapinarof [25,26].
Not yet the last word in wisdom
The itch-scratch circuit is the end of a cascade of complex interactions between environmental and exposure factors [14,32,33], the skin microbiome [15–19], the epidermal barrier [20,21], and immune and inflammatory responses [31]. These processes develop against a particular genetic and still poorly understood epigenetic background [22]. Whether it is directly inflammation-associated factors that contribute to the pathomechanism and severity of pruritus or other factors are involved has not yet been clarified. Although itching in moderate and severe atopic dermatitis has been observed to be closely associated with the severity of atopic dermatitis, many atopic dermatitis patients report pruritus that is not confined to the lesional skin, and some patients with mild atopic dermatitis suffer from severe itching [11,23,24].
Literature:
- Bonnekoh H, Butze M, Metz M: JDDG 2022; 20(2): 150-156.
- Kobyletzki LB, et al: Acta Derm Venereol 2017; 97(1): 86-90.
- Silverberg JI, et al: J Am Acad Dermatol 2020; 82(6): 1328-1336.
- Wollenberg A, et al: Br J Dermatol 2021; 184(3): 437-449.
- Silverberg JI, et al: Br J Dermatol 2021; 184(3): 450-463.
- Simpson EL, et al: Br J Dermatol 2020; 183(2): 242-255.
- Reich K, et al: JAMA Dermatol 2020; 156(12): 1333-1343.
- Guttman-Yassky E, et al: Lancet 2021; 397(10290): 2151-2168.
- Reich K, et al: Lancet 2021; 397(10290): 2169-2181.
- Bieber T: Nature Reviews Drug Discovery 2022; 21(1): 21-40.
- Huet F, et al: Acta Derm Venereol 2019; 99(3): 279-283.
- Kage P, Simon JC, Treudler R: J Dtsch Dermatol Ges 2020; 18(2): 93-102.
- Stand S: N Engl J Med 2021; 384(12): 1136-1143.
- Mack MR, Kim BS:Trends Immunol 2018; 39: 980-991.
- Byrd AL, Belkaid Y, Segre JA: Nature Reviews Drug Discovery 2018; 16: 143-155.
- Meisel JS, et al: Microbiome 2018; 6: 20.
- Kong HH, et al: Genome Res 2012; 22: 850-859.
- Shi B, et al: J Allergy Clin Immuno 2016; 138: 1233-1236.
- Williams MR, et al: Sci. Transl Med 2019;11:eaat8329.
- Kim BE, Leung DY: Allergy Asthma Immunol Res 2012; 4: 12-16.
- Rerknimitr P, et al: Inflammation and Regeneration 2017; 37: 14.
- Marenholz I, et al: Nature Communications 2015; 6: 8804.
- Hawro T, et al: J Am Acad Dermatol 2021; 84(3): 691-700.
- Chovatiya R, et al: Ann Allergy Asthma Immunol 2021; 127(1): 83-90e2.
- Jo CE, Gooderham M, Beecker J: Int J Dermatol 2022; 61(2): 139-147.
- Paller AS, et al: J Am Acad Dermatol 2021; 84(3): 632-638.
- Med ChemExpress, www.medchemexpress.com/lab-consumables-gift.html (last accessed May 27, 2022).
- Dillon SR, et al: Nature Immunology 2004; 5(7): 752-760.
- Zheng T, et al: J Invest Dermatol 2009; 129(3): 742-751.
- Langan SM, Irvine AD, Weidinger S: Lancet 2020; 396(10247): 345-360.
- Weidinger S, et al: Atopic dermatitis. Nature Reviews Disease Primers 2018; 4: 1.
- Gilles S, et al: Experimental Dermatology 2018; 27: 1193-1200.
- Stefanovic N, Flohr C, Irvine AD: Allergy 2020; 75: 63-74.
- Drug Information, www.swissmedicinfo.ch, (last accessed Jun. 12, 2022).
DERMATOLOGIE PRAXIS 2022; 32(3): 22-23